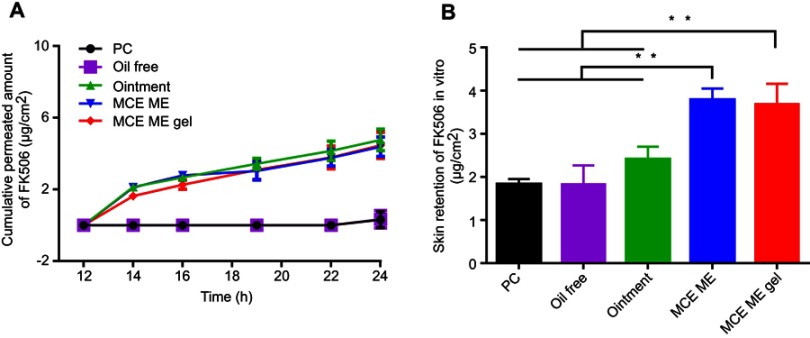
Figure 2.
In vitro skin penetration of FK506 from different formulations (A) and skin retention of FK506 after 24 h penetration (B).
Note: **P<0.001.
Abbreviations: FK506, tacrolimus; PC, The solution of 0.1% tacrolimus (FK506,w/v) dissolved in propylene carbonate; Oil free, 0.1% (w/v) FK506 solution containing the same surfactant and cosurfactant as microemulsion but without menthol/camphor eutectic; Ointment, 0.1%(w/w) FK506 commercial ointment; MCE ME, 0.1% (w/v) FK506 microemulsion based on menthol/camphor eutectic; MCE ME gel, 0.1% (w/w) FK506 microemulsion gel based on menthol/camphor eutectic; h, hours; SD, standard deviation.