Figure 2: Identification of mutation in human KCNJ6.
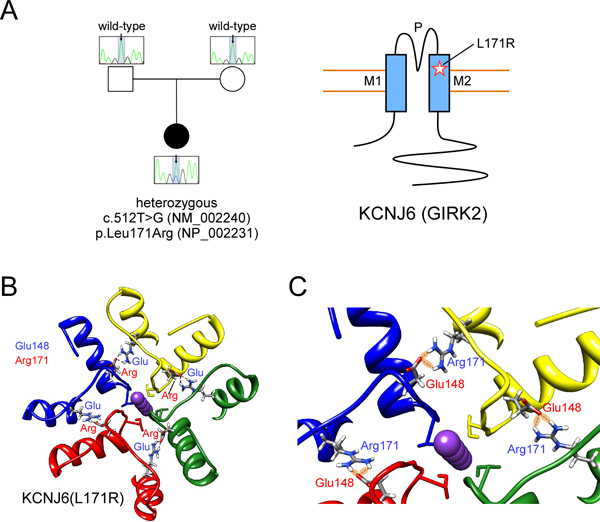
(A) Sequencing of patient and parent DNA revealed a mutation in KCNJ6. Cartoon shows position of L171R mutation in GIRK2 ion channel (M1, M2: transmembrane domains; P: pore). (B) 3D structural model of human GIRK2 obtained by homology modeling from mouse GIRK2 channel (PDB: 3SYO). The mutation L171R allows Arg171 to potentially form H-bonds with Glu148 in the adjacent subunit. This electrostatic interaction could produce a more stable structure and impair conformational movements, i.e., opening/closing of the channel, or alter ion selectivity. (C) A detail of the 3D structural model of human KCNJ6 shows the network of interactions (H-bonds) that stabilize the conformation of the outer part (the selectivity filter) of the KCNJ6 channel.
