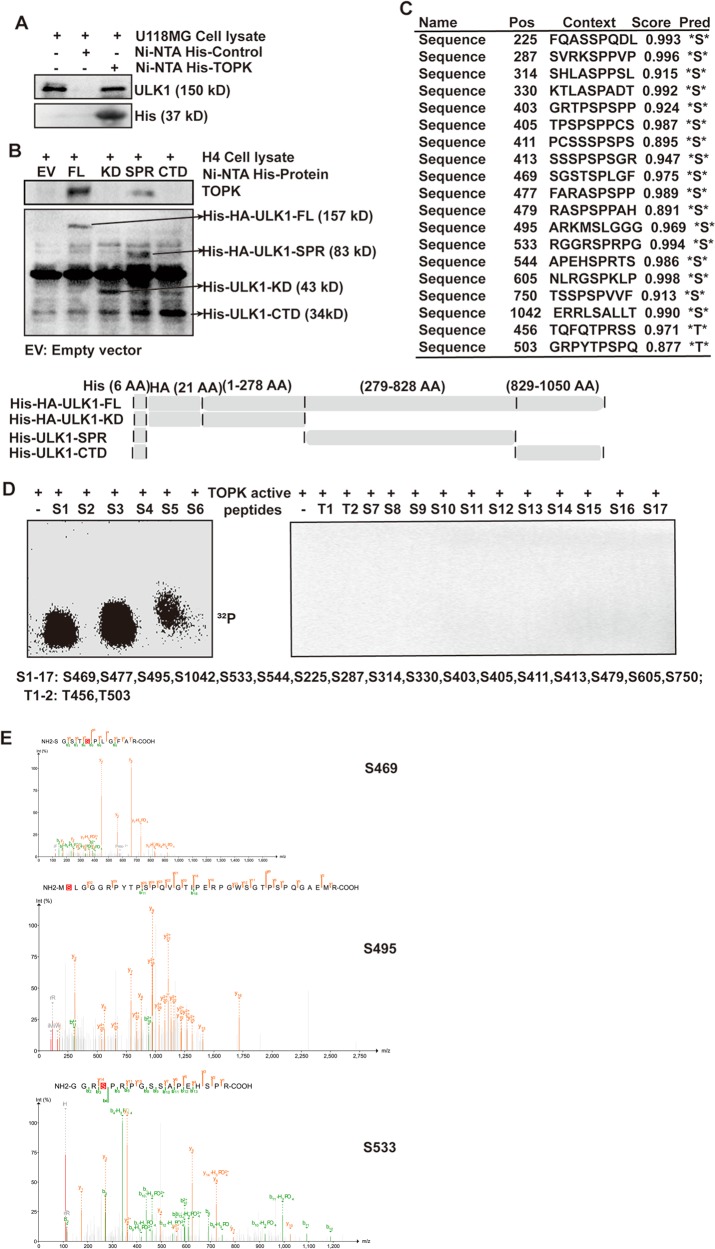
Fig. 5. TOPK directly binds with and phosphorylates ULK1 at Ser469, Ser495, and Ser533.
a TOPK directly bound with endogenous ULK1 of U118MG cells. Purified recombinant Ni-NTA His·Bind Resin-TOPK or Ni-NTA His·Bind Resin was mixed with U118MG cell lysate. Ni-NTA His·Bind Resin was used as a negative control. b Endogenous TOPK of H4 cells directly bound with ULK1-SPR. Purified recombinant Ni-NTA His·Bind Resin-ULK1-FL (full length), Ni-NTA His·Bind Resin-ULK1-KD (Kinase Domain), Ni-NTA His·Bind Resin-ULK1-SPR (Serine Proline-Rich), and Ni-NTA His·Bind Resin-ULK1-CTD (C-Terminal Domain) or Ni-NTA His·Bind Resin-EV(empty vector) was mixed with H4 cell lysate. Ni-NTA His·Bind Resin-EV was used as a negative control. Schematic diagram of ULK1 structure and eukaryotic expression construction of each domain was shown in the below. c NetPhos2.0 software program was used to predict the potential phosphorylation serine and threonine sites of ULK1. d TOPK phosphorylated ULK1 at Ser469, Ser495, and Ser533 in an in vitro kinase assay. TOPK served as an active kinase and ULK1 peptides as substrates. Active TOPK was incubated with each peptide in the presence of [γ-32P] ATP, followed by autoradiography. e A mass spectrometry (MS) assay was carried out to analyze the phosphorylation modification of ULK1 after an in vitro kinase assay in which TOPK served as an active kinase and His-ULK1-FL protein as substrate