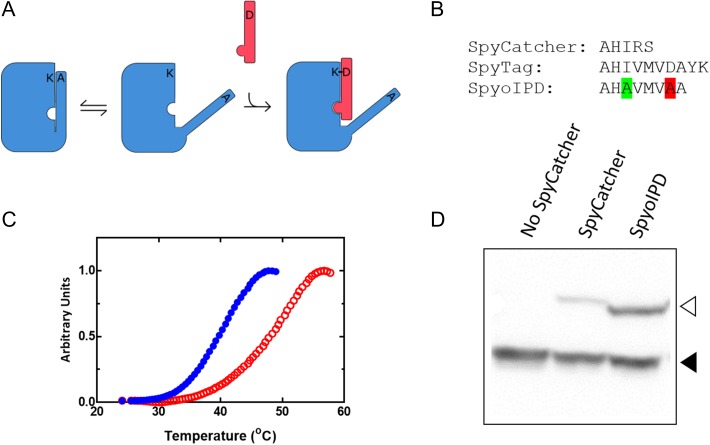
Fig. 2.
Design and properties of SpyoIPDs in vitro and in vivo. (A) To improve stability of the SpyCatcher protein (large rectangle with cutouts), we reintroduced portions of the C-terminal β-strand (thin rectangular ‘overhang’ attached to SpyCatcher) that was originally removed to make SpyTag. The reactive Asp on this extension was mutated to Ala (D556A) to prevent reaction with the Lys in the SpyCatcher region, and the appended sequence was also mutated (I552A) to weaken its interaction with the rest of the domain, allowing SpyTag (thin rectangle) to displace the reintroduced β-strand and react with SpyCatcher. (B) Comparison of the C-terminal sequences of SpyTag, SpyCatcher and SpyoIPD. The highlighted Ala second from the C-terminus replaces the isopeptide bond-forming Asp. The highlighted Ala six residues from the C-terminus replaces Ile 552 to weaken binding between SpyCatcher and the reintroduced sequence. (C) Differential scanning fluorimetry traces of SpyCatcher (solid circles) and SpyoIPD (hollow circles). (D) Comparison of the in vivo activity of SpyCatcher and SpyoIPD. SpyTag was expressed as a fusion to EGFP from a medium strength promoter on a low copy number plasmid. An N-terminal V5 epitope was also fused to EGFP to facilitate easy detection. A Western blot, probing for the V5 epitope, is shown. Lanes and bands are as labeled. The lower molecular weight band corresponds to unreacted EGFP-ST (filled triangle), and the higher molecular weight band to the covalent EGFP-ST-SC or EGFP-ST-SpyoIPD conjugate (hollow triangle).