Figure 1. Mechanisms of propagation for experimental evolution.
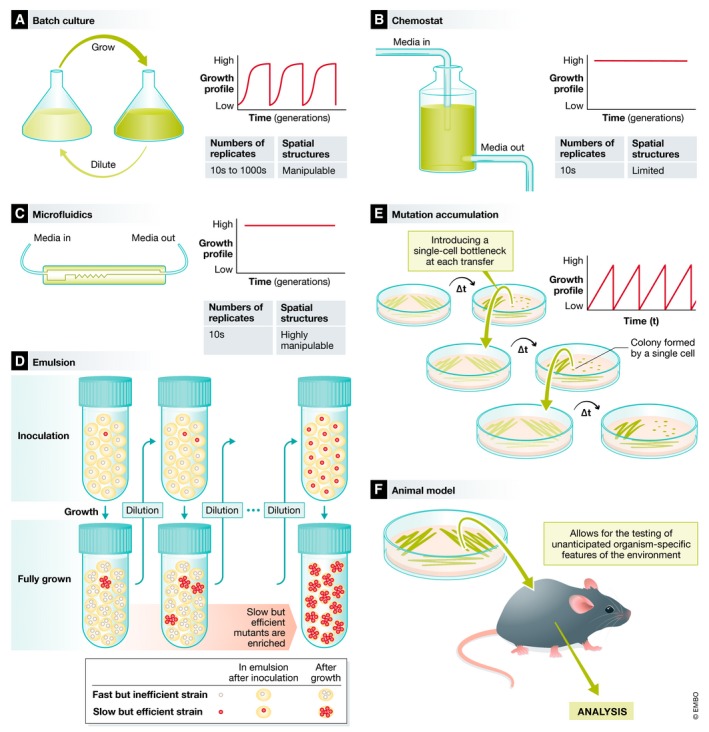
(A) Batch culture requires the regular dilution of culture into fresh media. These experiments are relatively easy to establish, since a range of vessels commonly used in a microbiology laboratory can be used for batch culture. These experiments can be scaled to a large number of replicates, for example when using 96‐well plates. (B) Chemostat culture systems include mechanisms for the constant supply of fresh medium. This provides for the continuous cultures of populations and constant growth without large fluctuations in populations size or growth phase. (C) Microfluidics provides the most precise control over the supply of media and supplements to cell cultures. Microfluidics may need to be custom designed, and the number of replicates will be limited. (D) Emulsion cultures take advantage of small cell‐containing vesicles that form when mixing an oil, surfactant and cells. The number of cells in each vesicle is determined by the ratio cell, surfactant and oil. The cells can be mixed back into a single population by vortexing and centrifuging the solution. One advantage of evolving cells in a large number of small populations is that this can select for yield per‐vesicle rather than rapid growth 144. (E) Mutation accumulation introduces a regular, single‐cell bottleneck into each replicate population. This achieved by streaking out cells on a petri dish and then choosing a single colony (founded by a single cell) to streak out the next plate. (F) Microbial cultures can be introduced into a model organism, often a plant or a mouse, and left to propagate for a number of generations before it is recovered from the organism. The recovered cells can be analysed or subjected to further propagation in the organism. This mode of experimental evolution allows for the testing of unanticipated organism‐specific features of the environment that are difficult to replicate in the laboratory.
