Abstract
Taste information from type III taste cells to gustatory neurons is thought to be transmitted via synapses. However, the molecular mechanisms underlying taste transduction through this pathway have not been fully elucidated. In this study, to identify molecules that participate in synaptic taste transduction, we investigated whether complexins (Cplxs), which play roles in regulating membrane fusion in synaptic vesicle exocytosis, were expressed in taste bud cells. Among four Cplx isoforms, strong expression of Cplx2 mRNA was detected in type III taste cells. To investigate the function of CPLX2 in taste transduction, we observed taste responses in CPLX2‐knockout mice. When assessed with electrophysiological and behavioral assays, taste responses to some sour stimuli in CPLX2‐knockout mice were significantly lower than those in wild‐type mice. These results suggested that CPLX2 participated in synaptic taste transduction from type III taste cells to gustatory neurons.
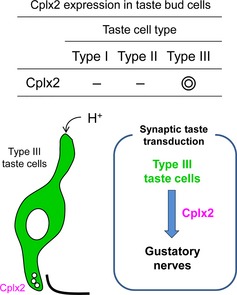
A part of taste information is thought to be transmitted via synapses. However, the molecular mechanisms have not been fully elucidated. To identify molecules that participate in synaptic taste transduction, we investigated complexins (Cplxs) expression in taste bud cells. Strong expression of Cplx2 mRNA was detected in taste bud cells. Furthermore, taste responses to some sour stimuli in CPLX2‐ knockout mice were significantly lower than those in wild‐type mice. These suggested that CPLX2 participated in synaptic taste transduction.
Keywords: CPLX2, sour, taste
Abbreviations used
- Cplx
complexin
- CT
chorda tympani
- CvP
circumvallate papillae
- DTA
diphtheria toxin
- FuP
fungiform papillae
- GL
glossopharyngeal
- IMP
inosine 5′‐monophosphate
- ISH
in situ hybridization
- KO
knockout
- MSG
monosodium glutamate
- NH4Cl
ammonium chloride
PKD
polycystic kidney disease
- TeNT
tetanus toxin
- TRP
transient receptor potential
- WT
wild type
Taste signaling begins with molecular events at modified epithelial‐derived taste cells, which are organized into taste buds in the oral cavity (Chandrashekar et al. 2006, 2010; Yarmolinsky et al. 2009). Mammalian taste bud cells are mainly classified into four cell types, each of which has distinct morphological and functional characteristics. Type IV taste cells, also termed ‘basal cells’, are subglobular progenitor cells that are thought to differentiate into the other three cell types (Miura et al. 2006). Types I, II, and III taste cells are mature cells that have a spindle shape and exhibit differences in the expression of taste‐related molecules, membrane potential, differentiation machinery, and synapse connections with gustatory nerves (Chandrashekar et al. 2006, 2010; Yarmolinsky et al. 2009).
Recent studies have identified taste receptors and taste signaling‐related molecules in taste bud cells; based on these studies, it has been hypothesized that different taste cells function to sense a part of five basic tastes, such as sweet, umami, bitter, sour, and salty. Sweet, umami, and bitter tastes are detected by type II taste cells, while sour taste is detected mainly by type III taste cells (Chandrashekar et al. 2006, 2010; Yarmolinsky et al. 2009). Although the perception of salty taste remains a matter of debate, it is generally accepted that the epithelial sodium channel‐expressing taste cells detect low concentrations of salt, whereas cells expressing bitter taste receptor T2Rs and sour taste receptor PKD2L1 detect high salt concentrations (Oka et al. 2013).
Taste information received by taste cells is transmitted to the gustatory nerves, including the chorda tympani (CT) and glossopharyngeal (GL) nerves. Using electron microscope analysis, conventional synaptic connections can be clearly observed between gustatory nerves and type III taste cells, but not between gustatory nerves and other types of taste cells (Murray 1973). In addition, it has been reported that type II taste cells use synapse‐independent and ion channel‐mediated ATP release for taste signal transmission (Chandrashekar et al. 2006, 2010; Yarmolinsky et al. 2009). Therefore, it is believed that type III taste cells possess the ability to perform taste transduction via conventional synapse connections. In fact, some SNARE proteins, such as synaptosomal‐associated protein 25 (SNAP‐25), syntaxin, and synaptobrevin 2, which are involved in synaptic exocytosis, have been reported to be expressed in type III taste cells (Yang et al. 2000, 2004, 2007; Ueda et al. 2006). However, because of the limited information available on the molecular characterization of taste bud cells, investigating the expression of synapse‐related molecules in taste bud cells is important for clarifying taste transduction mechanisms via synapses.
Complexins (Cplxs), a family of synapse‐related molecules, are cytosolic proteins in which 45% of the amino acid residues are charged. Cplxs bind to SNARE proteins through contact with the middle region (amino acids 48–70) (Ishizuka et al. 1995; McMahon et al. 1995; Takahashi et al. 1995) and facilitate spontaneous exocytosis (Maximov et al. 2009; Yang et al. 2010; Kaeser‐Woo et al. 2012). In mammals, four Cplx isoforms, Cplx1–4, exist (Reim et al. 2005; Kasai et al. 2012). Cplx1–3 is expressed in the brain (Reim et al. 2005). Cplx3 expression in the mouse brain is very low and does not significantly contribute to synaptic transmission (Xue et al. 2008). Cplx1 and Cplx2 are expressed in the olfactory bulb, striatum, hippocampus, thalamus, and cerebellar cortex in mouse (Ishizuka et al. 1999; Freeman and Morton 2004). In addition, all four isoforms are expressed in the retina, where Cplx1 and Cplx2 are found in conventional synapses of amacrine cells, and Cplx3 and Cplx4 are predominantly expressed in ribbon synapses of photoreceptors and bipolar cells (Reim et al. 2005). However, it remains unknown whether Cplx is involved in synaptic taste transduction.
In this study, to identify candidate molecules that participate in synaptic taste transduction, we investigated the expression of Cplxs in taste bud cells and used an in vivo CPLX2‐knockout (KO) mouse model to investigate the function of CPLX2 in taste transduction. Our study provided important insights into the involvement of CPLX2 in the synaptic taste transduction from type III taste cells to gustatory nerves.
Materials and methods
Materials
Citric acid, HCl, tartaric acid, NaCl, and sucrose were purchased from Kanto Chemical (Tokyo, Japan). Denatonium benzoate, monosodium glutamate (MSG), and inosine 5′‐monophosphate (IMP) were from Sigma (St. Louis, MO, USA). All other reagents were of analytical grade and were from standard suppliers.
Animals
All experiments were performed following the protocols approved by the animal care committee of the University of Tokyo. CPLX2‐KO mice (Takahashi et al. 1999), which were from the C57/B6 background, were supplied by the Center for Animal Resources and Development, Kumamoto University. Although CPLX2‐KO mice are viable and fertile, abnormalities in the plasticity of neural circuits have been reported (Takahashi et al. 1999; Huang et al. 2000; Reim et al. 2001; Gibson et al. 2005; Radyushkin et al.2010). Wild‐type (WT; Cplx2+/+) and homozygote KO (Cplx2−/−) mice were generated by breeding from heterozygote (Cplx2+/−) pairs of mice.
cDNA cloning and reverse transcription–polymerase chain reaction
cDNA fragments were obtained by reverse transcription–polymerase chain reaction (RT‐PCR) using total RNA from the circumvallate papillae (CvP), brain, retina, and testes of C57BL/6J mice (male, 12–30 weeks old). The epithelial tissue surrounding the taste buds was included in the CvP sample. A portion of the cDNA was used for standard PCR to detect Cplx1–4 and Trpm5, a representative taste marker molecule. PCR primers were designed as follows: Cplx1 (product size: 307 bp): forward primer, 5′‐GGAAAAGGACCCCGATGCTG‐3′; reverse primer, 5′‐CCCAGGCAGGTACTTGATGAC‐3′. Cplx2 (product size: 285 bp): forward primer, 5′‐AGGACCCAGACGCACAGAAG‐3′; reverse primer, 5′‐GCACTGTGTCCAGGATGCTC‐3′. Cplx3 (product size: 305 bp): forward primer, 5′‐CAACTGGTGGAGGAGAAGATGG‐3′; reverse primer, 5′‐GATTGCTTGAGGTCCCCTAGTG‐3′. Cplx4 (product size: 244 bp): forward primer, 5′‐TAGGATTTGGAGGTGGGTCTGAAG‐3′; reverse primer, 5′‐CCAGCCAGTTGGATTTGTGTCTC‐3′. Trpm5 (product size: 455 bp): forward primer, 5′‐CTGATCGCCATGTTCAGCTA‐3′; reverse primer, 5′‐ATGACGGATACACTGGCTCC‐3′. The following probe templates for Cplx family genes were obtained by RT‐PCR: Cplx1 (NM_007756, probe region: 114–2178), Cplx2 (NM_009946, probe region: 2811–4865), Cplx3 (NM_146223, probe region: 77–2810), and Cplx4 (NM_145493, probe region: 9–1593). The probes for taste marker molecules in In situ hybridization (ISH) were described previously (Ohmoto et al. 2008; Matsumoto et al. 2011). DNA fragments were cloned into the pBluescript SK vector (Stratagene, La Jolla, CA, USA) and sequenced using a 3130 Genetic Analyzer (Applied Biosystems, Foster City, CA, USA).
In situ hybridization
The CvP, fungiform papillae (FuP), and soft palate were removed from the tongues of C57BL/6J and CPLX2‐KO mice (male, > 7 weeks old) after cervical dislocation, and the tissues were embedded in OCT compound (Sakura Finetek, Tokyo, Japan). The embedded samples were snap‐frozen in liquid nitrogen and stored at −80°C until later use. ISH was performed as described previously (Ohmoto et al. 2008; Matsumoto et al. 2011). Briefly, digoxigenin‐ and fluorescein‐conjugated antisense RNAs were synthesized using a DIG RNA Labeling Mix (Roche Diagnostics, Indianapolis, IN, USA) and Fluorescein RNA Labeling Mix (Roche Diagnostics), respectively. The synthesized RNAs were used for hybridization after fragmentation to approximately 500 bases under alkaline conditions. For single labeling, signals were developed using alkaline phosphatase‐conjugated anti‐digoxigenin antibodies (Roche Diagnostics) and 4‐nitro blue tetrazolium chloride/5‐bromo‐4‐chloro‐3‐indolyl‐phosphate as chromogenic substrates. Stained sections were observed under an Olympus BX‐51 microscope (Olympus, Tokyo, Japan). For fluorescence double labeling, horseradish peroxidase‐conjugated anti‐fluorescein antibodies (Roche Diagnostics) and alkaline phosphatase‐conjugated anti‐digoxigenin antibodies (Roche Diagnostics) were used in combination with the TSA biotin system (Perkin Elmer, Norwalk, CT, USA), Alexa488‐conjugated streptavidin (Molecular Probes, Eugene, OR, USA), and an HNPP Fluorescent Detection Set (Roche Diagnostics). Fluorescent images were observed under an FV1000 confocal laser‐scanning microscope (Olympus).
Gustatory nerve recording
Whole gustatory nerve responses from CT and GL nerves were obtained as described previously (Matsumoto et al. 2011). The gustatory nerve response was measured in WT (n = 5) and CPLX2‐KO (n = 5–7) mice. As sour stimuli, 1–30 mM citric acid, 1–30 mM HCl, and 1–30 mM tartaric acid were used. As salty, sweet, bitter, and umami taste stimuli, 30–300 mM NaCl, 300 or 500 mM sucrose, 3 or 10 mM denatonium benzoate, and 100 mM MSG +0.5 mM IMP were used, respectively. The relative response magnitude for each tastant was calculated by comparing with that of 100 mM ammonium chloride (NH4Cl) as a control.
Behavioral assay
Behavior was assessed using the 48‐h two‐bottle preference test as described previously (Matsumoto et al. 2011). WT (n = 5–8) and CPLX2‐KO (n = 5–11) mice were used for the preference test. As sour stimuli, 0.3–30 mM citric acid, 0.3–30 mM HCl, and 0.3–30 mM tartaric acid were used. As the basic taste stimuli, 3–1000 mM NaCl, 3–30 mM sucrose, 0.03–1 mM denatonium benzoate, and 1–300 mM MSG + 0.5 mM IMP were used.
Statistical analysis
Data were expressed as the mean ± SEM. For all analyses, differences with p values of less than 0.05 were considered significant using Student's t‐test.
Results
Expression of Cplxs in taste buds of the CvP
To explore the possibility that Cplxs participate in the signaling pathway at taste bud cells, we first investigated the mRNA expression of Cplxs in taste bud cells. There are four Cplx isoforms, Cplx–4 (Reim et al. 2005; Kasai et al. 2012). Using RT‐PCR analysis, we showed that Cplx2 was detected in the CvP sample, while all Cplx isoforms were expressed in the retina (Fig. 1a). ISH analysis also indicated that an intense signal for Cplx2, but not for Cplx1, ‐3, or ‐4, was detected in the CvP, fungiform papillae, and soft palate (Fig. 1b).
Figure 1.
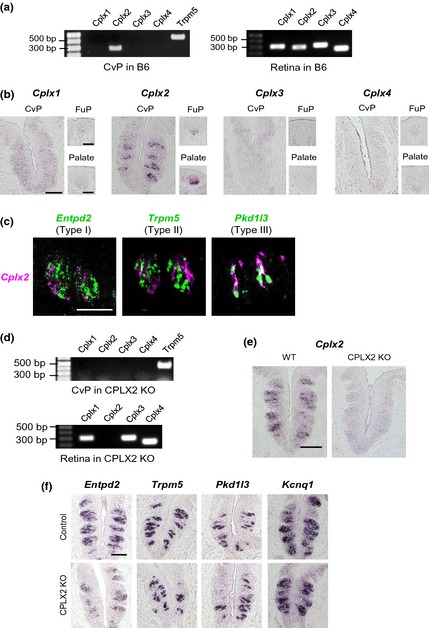
Expression of complexin (Cplx) family members. (a) RT‐PCR of Cplx family mRNAs (Cplx1–Cplx4) in the circumvallate papillae (CvP) and retina of C57BL/6J (B6) mice. (b) In situ hybridization (ISH) of Cplx family mRNAs (Cplx1–Cplx4) in the CvP, fungiform papillae (FuP), and soft palate (Palate) of B6 mice. (c) Double labeled ISH of Cplx2 (magenta) and taste marker molecules (green) in the CvP of B6 mice. Entpd2, Trpm5, and Pkd1l3 were used as markers of type I, II, and III cells, respectively. (d) RT‐PCR of Cplx family mRNAs (Cplx1–Cplx4) in the CvP and retina of CPLX2‐knockout (KO) mice. (e) ISH of Cplx2 mRNA in wild‐type (WT) and CPLX2‐KO mice. (f) ISH of taste marker molecules in control (C57BL/6J or WT) and CPLX2 KO mice. Type I taste cell marker: Entpd2. Type II taste cell markers: Trpm5. Type III taste cell markers: Pkd1l3. Taste bud marker: Kcnq1. Signals for all marker molecules were observed in CPLX2‐KO mice. Scale bars show 50 μm in the CvP and 20 μm in the FuP and Palate.
To determine which cell types expressed Cplx2, we performed double labeled ISH with taste cell marker molecules (Fig. 1c). In the CvP of WT mice, the Cplx2 signal overlapped with that of the type III marker Pkd1l3, although it did not overlap with the signal for the type I marker Entpd2 or the type II marker Trpm5. Ninety‐five percent of Cplx2‐positive cells exhibited Pkd1l3 signal (37 of 39 cells in total 293 taste bud cells). On the other hand, 77% of Pkd1l3 positive cells exhibited Cplx2 signal (37 of 48 cells in total 293 taste bud cells). These findings indicated that Cplx2 was the only Cplx expressed in taste bud cells and that Cplx2 was specifically expressed in type III taste cells.
Expression of taste marker molecules in the taste bud cells of CPLX2‐KO mice
The role of CPLX2 in the reception and transmission of taste information was investigated using CPLX2‐KO mice. First, we examined the expression of Cplx2 and other Cplx family molecules. ISH and RT‐PCR showed that Cplx2 mRNA was not detected in the CvP in CPLX2‐KO mice (Fig. 1d and e). Then, we assessed the mRNA expression of taste marker molecules in CPLX2‐KO mice (Fig. 1f). Signals for the marker for Entpd2, Trpm5, Pkd1l3, and Kcnq1 which is all taste cell marker were clearly observed in both WT and CPLX2‐KO mice. Thus, although representative taste marker molecules were expressed in the taste bud cells of CPLX2‐KO mice, Cplxs were not.
Assessment of the taste response of CPLX2‐KO mice by electrophysiological and behavioral assays
Since representative taste marker molecules were observed in the taste bud cells of CPLX2‐KO mice, we evaluated the taste responses of CPLX2‐KO mice using electrophysiological and behavioral assays. We measured the gustatory nerve responses to the five basic tastes. In CPLX2‐KO mice, CT responses to 3 and 10 mM citric and 1, 3, and 30 mM tartaric acid were significantly reduced (Fig. 2a–c). Although the CT responses to HCl in KO mice were not significantly reduced, they were smaller than those in the WT mice. A significant reduction in GL responses to 1 mM citric acid, 3 and 30 mM HCl, and 10 mM tartaric acid was observed (Fig. 3a–c). However, there was no significant difference between WT and CPLX2‐KO mice in the CT and GL responses to sucrose, MSG+IMP, and denatonium (Figs 2e and 3e). Therefore, CPLX2 deficiency significantly reduced the gustatory nerve responses to sour stimuli, although the effect was modest.
Figure 2.
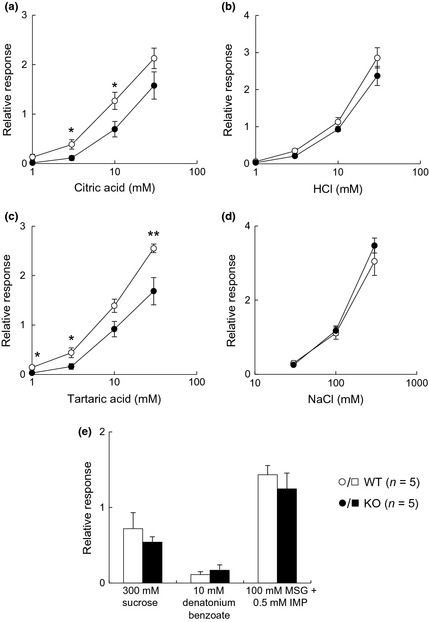
Chorda tympani (CT) nerve responses (relative to 100 mM NH 4Cl) to taste stimuli in wild‐type (WT) and CPLX2‐knockout (KO) mice. Integral responses for (a) citric acid, (b) HCl, (c) tartaric acid, (d) NaCl, and (e) sucrose, denatonium benzoate, and monosodium glutamate (MSG)+inosine 5′‐monophosphate (IMP). *p < 0.05 and **p < 0.03 (t‐test).
Figure 3.
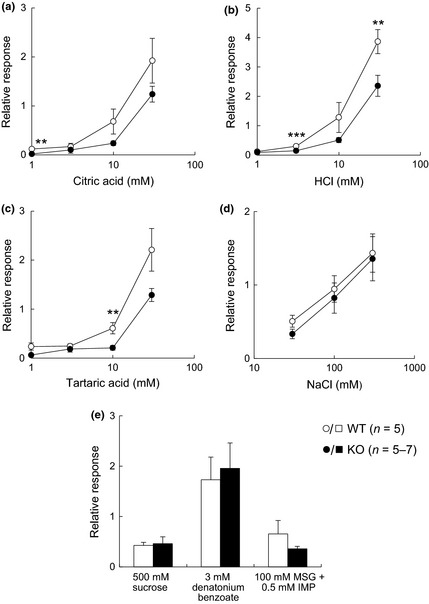
Glossopharyngeal (GL) nerve responses (relative to 100 mM NH 4Cl) to taste stimuli in wild‐type (WT) and CPLX2‐knockout (KO) mice. Integral responses for (a) citric acid, (b) HCl, (c) tartaric acid, (d) NaCl, and (e) sucrose, denatonium benzoate, and monosodium glutamate (MSG)+inosine 5′‐monophosphate (IMP). *p < 0.05, **p < 0.03 and ***p < 0.01 (t‐test).
Furthermore, to investigate the effects of CPLX2 KO on behavioral characteristics, we examined the changes in palatability of basic tastes using a 48‐h two‐bottle preference test. Although the preference for citric acid did not significantly differ between the WT and KO mice, CPLX2‐KO mice exhibited a slight reduced avoidance of 3 and 10 mM HCl and 10 mM tartaric acid that was significant (Fig. 4a–c). However, there was no significant difference between WT and CPLX2‐KO mice in the palatability of sweet, umami, and bitter stimuli under our experimental conditions (Fig. 4d–g). Therefore, we concluded that CPLX2 deficiency mainly affected sour taste palatability, although the effect was modest.
Figure 4.
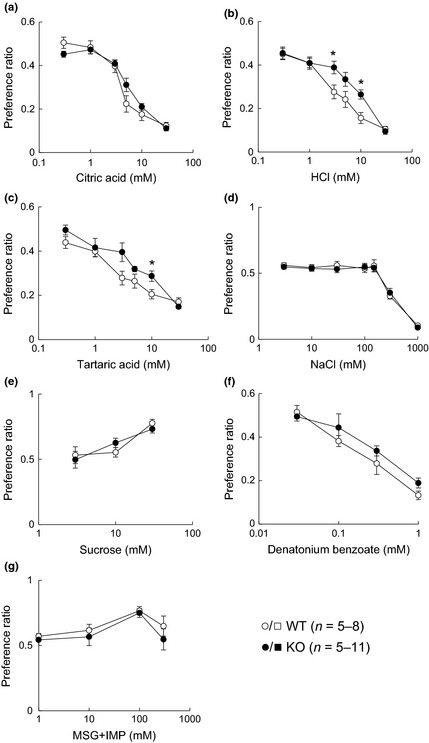
Taste preferences of wild‐type (WT) and CPLX2‐knockout (KO) mice for different tastants in 48‐h two‐bottle preference tests. Preference ratios for (a) citric acid, (b) HCl, (c) tartaric acid, (d) NaCl, (e) sucrose, (f) denatonium benzoate, and (g) monosodium glutamate (MSG)+IMP. *p < 0.05 (t‐test).
Recently, type III taste cells have been reported to participate in recognition of high concentrations of salt (Oka et al. 2013). Because neural responses and avoidance to sour tastes, which are received by type III taste cells, were decreased in CPLX2‐KO mice, we also investigated taste responses to NaCl stimuli. However, significant differences in neural responses (Figs 2d and 3d) and behavioral responses (Fig. 4d) to high concentrations of NaCl between WT and CPLX2‐KO mice were not detectable under our experimental conditions.
Discussion
Despite previous efforts to study signal transduction mechanisms in peripheral taste organs, the details of these mechanisms have not yet been fully elucidated. However, the characteristics of each taste cell type have been identified as related to a specific expression pattern of taste‐related molecules (Chandrashekar et al. 2006, 2010; Yarmolinsky et al. 2009). Type III taste cells express some synapse‐related molecules as well as polycystic kidney disease (PKD) 2L1/PKD1L3 (Yang et al. 2000, 2004, 2007; Huang et al. 2006; Ishimaru et al. 2006; Ueda et al. 2006). In addition, type III taste cells form characteristic conventional synapses with gustatory nerves, whereas other taste cells do not. The identification of synapse‐related molecules is useful for investigating the mechanisms underlying taste transduction from type III taste cells. Type II taste cells lack conventional synaptic structures. However, it was recently reported that information from type II taste cells is transmitted to gustatory neural circuits by non‐vesicular ATP release through the calcium homeostasis modulator 1 ion channel (Taruno et al. 2013). On the other hand, molecular mechanisms transmitting the taste information from type III taste cells have not been fully elucidated; this will be important for uncovering the mechanisms of taste signal transduction to the central nervous system.
In this study, we focused on the synapse‐related molecules, Cplxs, and found that only Cplx2 mRNA was expressed specifically in type III taste cells (Fig. 1), suggesting the participation of CPLX2 in taste transduction via type III taste cells. When phenotyping analyses of taste reception were carried out using CPLX2‐KO mice, the neural response to sour stimuli was significantly lower in CPLX2‐KO mice than in WT mice, although the nerve responses to sweet, bitter, umami, and salty stimuli were not changed between WT and KO mice (Figs 2 and 3). These results were consistent with the reduced avoidance to sour tastes and the observation that there were no significant differences in the preference for sweet, bitter, umami, and salty stimuli observed in mice lacking CPLX2 (Fig. 4). Because CPLX2 generally regulates synaptic exocytosis (Maximov et al. 2009; Yang et al. 2010; Kaeser‐Woo et al. 2012), these results suggest that CPLX2 actually participates in the synaptic transduction from type III cells to gustatory neurons. Multiple neurotransmitters have been reported to participate in synaptic events in peripheral taste organs (Chandrashekar et al. 2006, 2010; Yarmolinsky et al. 2009). Type III taste cells release serotonin in an activity‐dependent manner (Huang et al. 2005). It will be necessary to investigate whether the lack of CPLX2 influences the release of neurotransmitter from taste cells.
Abnormal transduction of sour stimuli has been reported in genetically modified mice, including PKD2L1‐diphtheria toxin (DTA) mice (Huang et al. 2006), PKD2L1‐TeNT mice (Chandrashekar et al. 2009), which express tetanus toxin (TeNT) in PKD2L1‐expressing cells, and PKD2L1/PKD1L3 double‐KO mice (Horio et al. 2011). PKD2L1‐DTA mice, whose type III taste cells were genetically ablated by targeted expression of DTA, completely lack the gustatory nerve responses to sour stimuli (Huang et al. 2006; Chandrashekar et al. 2009). PKD2L1‐TeNT mice, in which TeNT is expressed in type III taste cells, also lack the gustatory nerve responses to sour stimuli (Chandrashekar et al. 2010). Although the CT nerve responses to sour stimuli are reduced in PKD2L1/PKD1L3 double‐KO mice, much of the response remains (Horio et al. 2011). Changes in the gustatory nerve responses to sour tastes in genetically modified mice have been reported as described above; however, changes in behavioral responses to sour tastes have not been reported. It should be noted that only a portion of the sour responses were decreased in our experimental conditions (Fig. 2 to 4). Both CPLX1 and CPLX2 are expressed in the central nervous system (Reim et al. 2005). Since Cplx1 mRNA was not detected by RT‐PCR or ISH in taste bud cells in the CvP (Fig. 1, 2), it is unlikely that CPLX1 compensates for the loss of CPLX2. On the other hand, Cplxs are thought to facilitate spontaneous exocytosis (Maximov et al. 2009; Yang et al. 2010; Kaeser‐Woo et al. 2012). Therefore, taste information from type III taste cells may be able to be transduced to peripheral gustatory nerves, even in the absence of CPLX2. However, reports have shown that CPLX2 KO does not result in a complete inhibition of the physiological functions of CPLX2. For example, the excitatory post‐synaptic currents in cortical and olfactory bulb neurons still remain in mice lacking both Cplx1 and Cplx2 (Yang et al. 2013). Moreover, other sour sensors, such as TRPV1, may produce the remaining sour responses in CPLX2‐KO mice. TRPV1, a member of the transient receptor potential (TRP) family, is activated by capsaicin, temperature, and sourness (Caterina et al. 1997). A subset of neurons in the petrosal ganglion, which contains cell bodies of GL nerves, express Trpv1 (Ohmoto et al. 2008; Matsumoto et al. 2011), indicating that GL nerves can be activated by sour substances. Therefore, sour information could be transferred to the peripheral gustatory and somatosensory systems, even when CPLX2 is absent from type III taste cells.
In addition, Cplx2 is expressed in the pons, medulla, and cerebral cortex (Ishizuka et al. 1999). Centers for taste perception such as the nucleus of the solitary tract and the gustatory cortex exist in these regions (Carleton et al. 2010). However, no differences in the perception of basic tastes other than the sour taste were observed in this study (Figs 2, 3, 4), suggesting that the difference in sour taste perception was caused by Cplx2 deficiency in the peripheral taste tissue.
In conclusion, in this study, we identified the expression of Cplx2 in type III taste cells and showed the involvement of CPLX2 in the transduction of taste information from type III taste cells to the gustatory neurons. These data provide important insights into the role of CPLX2 in taste sensation.
Acknowledgments and conflict of interest disclosure
We thank Dr Hideto Kaba (Kochi Medical School, Japan) for providing CPLX2‐KO mice and Ms Taeko Fukuda (the University of Tokyo, Japan) for supporting animal care. This work was supported, in part, by the Funding Program for Next Generation World‐Leading Researchers from the Japan Society for the Promotion of Science (LS037 to TM), by a Grant‐in‐Aid for Young Scientists (A) (26712014 to MN) in the Priority Area Food Science from the Japan Society for the Promotion of Science, and by Council for Science, Technology and Innovation (CSTI), Cross‐ministerial Strategic Innovation Promotion Program (SIP), ‘Technologies for creating next‐generation agriculture, forestry and fisheries’.
All experiments were conducted in compliance with the ARRIVE guidelines. The authors have no conflict of interest to declare.
References
- Carleton A., Accolla R. and Simon S. A. (2010) Coding in the mammalian gustatory system. Trends Neurosci. 33, 326–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina M. J., Schumacher M. A., Tominaga M., Rosen T. A., Levine J. D. and Julius D. (1997) The capsaicin receptor: a heat‐activated ion channel in the pain pathway. Nature 389, 816–824. [DOI] [PubMed] [Google Scholar]
- Chandrashekar J., Hoon M. A., Ryba N. J. P. and Zuker C. S. (2006) The receptors and cells for mammalian taste. Nature 444, 288–294. [DOI] [PubMed] [Google Scholar]
- Chandrashekar J., Yarmolinsky D., von Buchholtz L., Oka Y., Sly W., Ryba N. J. P. and Zuker C. S. (2009) The taste of carbonation. Science 326, 443–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrashekar J., Kuhn C., Oka Y., Yarmolinsky D. A., Hummler E., Ryba N. J. P. and Zuker C. S. (2010) The cells and peripheral representation of sodium taste in mice. Nature 464, 297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman W. and Morton A. J. (2004) Differential messenger RNA expression of complexins in mouse brain. Brain Res. Bull. 63, 33–44. [DOI] [PubMed] [Google Scholar]
- Gibson H. E., Reim K., Brose N., Morton A. J. and Jones S. (2005) A similar impairment in CA3 mossy fibre LTP in the R6/2 mouse model of Huntington's disease and in the complexin II knockout mouse. Eur. J. Neurosci. 22, 1701–1712. [DOI] [PubMed] [Google Scholar]
- Horio N., Yoshida R., Yasumatsu K., Yanagawa Y., Ishimaru Y., Matsunami H. and Ninomiya Y. (2011) Sour taste responses in mice lacking PKD channels. PLoS ONE 6, e20007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G. Z., Ujihara H., Takahashi S., Kaba H., Yagi T. and Inoue S. (2000) Involvement of complexin II in synaptic plasticity in the CA1 region of the hippocampus: the use of complexin II‐lacking mice. Jpn. J. Pharmacol. 84, 179–187. [DOI] [PubMed] [Google Scholar]
- Huang Y. J., Maruyama Y., Lu K. S., Pereira E., Plonsky I., Baur J. E., Wu D. Q. and Roper S. D. (2005) Mouse taste buds use serotonin as a neurotransmitter. J. Neurosci. 25, 843–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. L., Chen X. K., Hoon M. A., Chandrashekar J., Guo W., Trankner D., Ryba N. J. P. and Zuker C. S. (2006) The cells and logic for mammalian sour taste detection. Nature 442, 934–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimaru Y., Inada H., Kubota M., Zhuang H., Tominaga M. and Matsunami H. (2006) Transient receptor potential family members PKD1L3 and PKD2L1 form a candidate sour taste receptor. Proc. Natl Acad. Sci. USA 103, 12569–12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuka T., Saisu H., Odani S. and Abe T. (1995) Synaphin: a protein associated with the docking/fusion complex in presynaptic terminals. Biochem. Biophys. Res. Commun. 213, 1107–1114. [DOI] [PubMed] [Google Scholar]
- Ishizuka T., Saisu H., Odani S., Kumanishi T. and Abe T. (1999) Distinct regional distribution in the brain of messenger RNAs for the two isoforms of synaphin associated with the docking/fusion complex. Neuroscience 88, 295–306. [DOI] [PubMed] [Google Scholar]
- Kaeser‐Woo Y. J., Yang X. F. and Sudhof T. C. (2012) C‐terminal complexin sequence is selectively required for clamping and priming but not for Ca2+ triggering of synaptic exocytosis. J. Neurosci. 32, 2877–2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai H., Takahashi N. and Tokumaru H. (2012) Distinct initial SNARE configurations underlying the diversity of exocytosis. Physiol. Rev. 92, 1915–1964. [DOI] [PubMed] [Google Scholar]
- Matsumoto I., Ohmoto M., Narukawa M., Yoshihara Y. and Abe K. (2011) Skn‐1a (Pou2f3) specifies taste receptor cell lineage. Nat. Neurosci. 14, 685–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maximov A., Tang J., Yang X. F., Pang Z. P. P. and Sudhof T. C. (2009) Complexin controls the force transfer from SNARE complexes to membranes in fusion. Science 323, 516–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon H. T., Missler M., Li C. and Sudhof T. C. (1995) Complexins: cytosolic proteins that regulate SNAP receptor function. Cell 83, 111–119. [DOI] [PubMed] [Google Scholar]
- Miura H., Kusakabe Y. and Harada S. (2006) Cell lineage and differentiation in taste buds. Arch. Histol. Cytol. 69, 209–225. [DOI] [PubMed] [Google Scholar]
- Murray R. G. (1973) The ultrastructure of taste buds, in Ultrastructure of sensory organs (Friedmann I., ed.), pp. 1–81. North Holland, Amsterdam. [Google Scholar]
- Ohmoto M., Matsumoto I., Yasuoka A., Yoshihara Y. and Abe K. (2008) Genetic tracing of the gustatory and trigeminal neural pathways originating from T1R3‐expressing taste receptor cells and solitary chemoreceptor cells. Mol. Cell Neurosci. 38, 505–517. [DOI] [PubMed] [Google Scholar]
- Oka Y., Butnaru M., von Buchholtz L., Ryba N. J. and Zuker C. S. (2013) High salt recruits aversive taste pathways. Nature 494, 472–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radyushkin K., El‐Kordi A., Boretius S. et al (2010) Complexin2 null mutation requires a ‘second hit’ for induction of phenotypic changes relevant to schizophrenia. Genes Brain Behav. 9, 592–602. [DOI] [PubMed] [Google Scholar]
- Reim K., Mansour M., Varoqueaux F., McMahon H. T., Sudhof T. C., Brose N. and Rosenmund C. (2001) Complexins regulate a late step in Ca2+‐dependent neurotransmitter release. Cell 104, 71–81. [DOI] [PubMed] [Google Scholar]
- Reim K., Wegmeyer H., Brandstatter J. H., Xue M. S., Rosenmund C., Dresbach T., Hofmann K. and Brose N. (2005) Structurally and functionally unique complexins at retinal ribbon synapses. J. Cell Biol. 169, 669–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S., Yamamoto H., Matsuda Z. et al (1995) Identification of two highly homologous presynaptic proteins distinctly localized at the dendritic and somatic synapses. FEBS Lett. 368, 455–460. [DOI] [PubMed] [Google Scholar]
- Takahashi S., Ujihara H., Huang G. Z., Yagyu K., Sanbo M., Kaba H. and Yagi T. (1999) Reduced hippocampal LTP in mice lacking a presynaptic protein: complexin II. Eur. J. Neurosci. 11, 2359–2366. [DOI] [PubMed] [Google Scholar]
- Taruno A., Vingtdeux V., Ohmoto M. et al (2013) CALHM1 ion channel mediates purinergic neurotransmission of sweet, bitter and umami tastes. Nature 495, 223–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda K., Ichimori Y., Okada H., Honma S. and Wakisaka S. (2006) Immunolocalization of SNARE proteins in both type II and type III cells of rat taste buds. Arch. Histol. Cytol. 69, 289–296. [DOI] [PubMed] [Google Scholar]
- Xue M. S., Stradomska A., Chen H. M., Brose N., Zhang W. Q., Rosenmund C. and Reim K. (2008) Complexins facilitate neurotransmitter release at excitatory and inhibitory synapses in mammalian central nervous system. Proc. Natl Acad. Sci. USA 105, 7875–7880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R., Crowley H. H., Rock M. E. and Kinnamon J. C. (2000) Taste cells with synapses in rat circumvallate papillae display SNAP‐25‐like immunoreactivity. J. Comp. Neurol. 424, 205–215. [DOI] [PubMed] [Google Scholar]
- Yang R. B., Stoick C. L. and Kinnamon J. C. (2004) Synaptobrevin‐2‐like immunoreactivity is associated with vesicles at synapses in rat circumvallate taste buds. J. Comp. Neurol. 471, 59–71. [DOI] [PubMed] [Google Scholar]
- Yang R. B., Ma H. Z., Thomas S. M. and Kinnamon J. C. (2007) Immunocytochemical analysis of syntaxin‐1 in rat circumvallate taste buds. J. Comp. Neurol. 502, 883–893. [DOI] [PubMed] [Google Scholar]
- Yang X. F., Kaeser‐Woo Y. J., Pang Z. P. P., Xu W. and Sudhof T. C. (2010) Complexin clamps asynchronous release by blocking a secondary Ca2+ sensor via its accessory α helix. Neuron 68, 907–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X. F., Cao P. and Sudhof T. C. (2013) Deconstructing complexin function in activating and clamping Ca2+‐triggered exocytosis by comparing knockout and knockdown phenotypes. Proc. Natl Acad. Sci. USA 110, 20777–20782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarmolinsky D. A., Zuker C. S. and Ryba N. J. P. (2009) Common sense about taste: from mammals to insects. Cell 139, 234–244. [DOI] [PMC free article] [PubMed] [Google Scholar]


