Scheme 2.
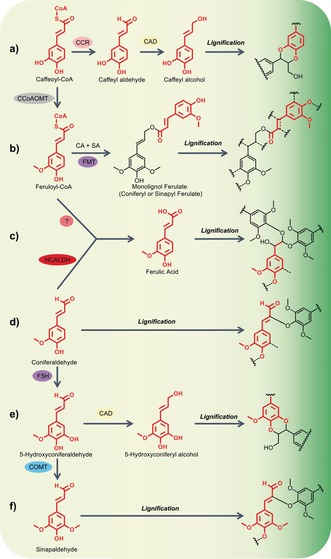
Incorporation of non‐native monomers in the lignification process, via genetic modification to the phenylpropanoid biosynthetic pathway (Scheme 1, see asterisked intermediates that are either the new monomers themselves, or give rise to a new monomer via the subsequent transformations shown here). The incorporation of new monomers (highlighted in red) by the same radical coupling modes gives rise to new structures in the lignin polymer (Table 3), mainly as a result of new opportunities for rearomatisation of the intermediate quinone methides: a) In CCoAOMT‐deficient softwoods, caffeyl alcohol is incorporated into the lignins producing benzodioxane structures. Some plants, such as vanilla, make lignin in their seed coats entirely from caffeyl alcohol, producing a polymer that is almost entirely composed of long chains of benzodioxane units. b) Employing a novel monolignol transferase enzyme, FMT, Feruloyl‐CoA can be conjugated to a monolignol, coniferyl alcohol (CA) or sinapyl alcohol (SA), to produce monolignol ferulate conjugates. Formation of labile ester linkages in the lignin polymer backbone can be achieved via lignification with a proportion of its monomer pool as these monolignol ferulate conjugates. Conjugates of this type are incorporated into the lignin structure in the same manner as conventional monomers.1 c) Ferulic acid itself can be formed either via the action of the enzyme HCALDH or by downregulation of CCR (Scheme 1). During lignification, double‐β‐O‐4‐coupling is undergone, to yield acid‐labile acetal bonds in the lignin. d) The intermediate, coniferaldehyde, accumulates in CAD‐deficient plants and can be incorporated into lignins via β‐O‐4‐coupling (shown) as well as other modes (not shown); the β‐O‐4‐coupling product is unsaturated due to the acidity of the β‐proton in the quinone methide intermediate that allows rearomatisation via H‐abstraction. e) In COMT‐deficient angiosperms, the intermediate 5‐hydroxyconiferaldehyde that accumulates can still be reduced by CAD, producing 5‐hydroxyconiferyl alcohol that, like caffeyl alcohol, produces benzodioxane analogues in the lignin. f) Sinapaldehyde also incorporates into lignins in CAD‐deficient angiosperms.
