Abstract
The transgenic rodent mutation assay was used to compare the dose–response relationship of lacZ mutant frequency (MF) in spermatogonial stem cells exposed acutely or subchronically to N‐ethyl‐N‐nitrosourea (ENU). Muta™Mouse males were exposed orally to 0, 25, 50, or 100 mg/kg ENU for acute exposures and 0, 1, 2, or 5 mg/(kg day) for 28‐day subchronic exposures. LacZ MF was measured in sperm collected 70 days post‐exposure to target spermatogonial stem cells. Dose–response data were fit to linear, quadratic, exponential, or power models. Acute exposure resulted in a dose‐dependent increase in MF that was significant (P < 0.05) at all doses tested and was best described by a quadratic dose–response model that was linear in the low dose range. In contrast, similar total doses fragmented over a 28‐day subchronic exposure only resulted in a significant increase in lacZ MF at the highest dose tested. Therefore, the subchronic no observable genotoxic effect level (NOGEL) was 2 mg/(kg day) (or 56 mg/kg total dose). The subchronic dose–response was best described by the exponential and power models, which were sublinear in the low dose range. Benchmark dose lower confidence limits (BMDLs) for acute and subchronic exposure were 3.0 and 1.0 mg/(kg day) (or 27.4 mg/kg total dose), respectively. These findings are supportive of a saturable DNA repair mechanism as the mutagenic mode of action for ENU in spermatogonia and imply that sufficiently low exposures would not cause appreciable genotoxic effects over background. This may have important implications for the quantitative risk assessment of germ cell mutagens. Environ. Mol. Mutagen. 56:347–355, 2015. © 2015 The Authors. Environmental and Molecular Mutagenesis Published by Wiley Periodicals, Inc.
Keywords: germ cell mutation, spermatogonia, transgenic rodent mutation assay, N‐ethyl‐N‐nitrosourea, dose–response modeling, threshold
INTRODUCTION
Germ cell mutations may be inherited and cause adverse health outcomes in unexposed offspring. Recent evidence has shown that the majority of de novo mutations are inherited from the male germline [Conrad et al., 2011], and that the number of mutations increases with paternal age [Kong et al., 2012]. These observations have led to an increased concern regarding the impact of environmental factors on the genetic integrity of the male germline, and as such, there has been elevated interest in the detection and characterization of male germ cell mutagens [Demarini, 2012]. However, the paucity of effective and economical tools has hindered the identification and comprehensive evaluation of such agents.
The transgenic rodent (TGR) mutation assay is a method recently endorsed by the international community [OECD, [Link]] for testing the ability of chemicals to induce DNA mutations in vivo, including those that occur in the germline. Because the TGR assay can detect mutations directly in sperm, it provides a practical, cost effective, and sensitive means for identifying chemicals that affect germ cells. While TGR germline mutation data are becoming more common (a detailed review in 2009 [OECD, [Link]] enumerated 257 records of TGR studies that investigated mutational effects in germ cells) most studies employ high dose acute exposures to known mutagens.
An aspect of emerging importance for mutagen characterization is to describe the dose–response relationship. Dose–response data for genotoxic agents often shed light on the molecular mode‐of‐action (MOA) [Julien et al., 2009] and may have potential applications in quantitative risk assessment [Muller et al., 2009; Pottenger and Gollapudi, 2010; Gollapudi et al., 2013; Cao et al., 2014; Johnson et al., 2014]. There is increasing evidence supporting the existence of a sublinear response for several DNA‐reactive mutagens, particularly for DNA‐alkylating agents such as methylnitrosourea, ethyl methanesufonate (EMS), and N‐ethyl‐N‐nitrosourea (ENU) [Doak et al., 2007; Gocke and Muller, 2009; Pottenger et al., 2009; Bryce et al., 2010; Lynch et al., 2011]. The mechanism responsible for the sublinear response is believed to involve DNA repair, where the repair machinery is sufficiently protective at low doses, but becomes saturated and overwhelmed as dose increases [Noveroske et al., 2000; Zair et al., 2011; Johnson et al., 2012; Thomas et al., 2013]. Although several studies have characterized the dose–response of several mutagens in in vitro and in vivo somatic cell systems, very few have examined the dose–response for alkylating agents in the germline. To our knowledge, no studies have examined dose–response relationships in the germline using the TGR assay following the OECD‐recommended 28‐day subchronic exposure regimen, nor have any studies compared the response between subchronic and acute exposure regimens using this assay in germ cells.
Here we employed the TGR mutation assay to measure the induced lacZ mutant frequency (MF) in sperm originating from spermatogonial stem cells exposed acutely or subchronically to the DNA‐alkylating agent ENU. Mutations induced in spermatogonial stem cells represent permanent effects in the germline and are thus a genotoxic endpoint of high biological significance and concern. We then performed model fitting to characterize the dose–response relationship between lacZ MF and ENU exposure, and compared the responses between acute and subchronic exposure regimens.
MATERIALS AND METHODS
Animal Treatment
All animal experiments were performed using protocols approved by Health Canada's Animal Care Committee. We used the Muta™Mouse transgenic mouse model (8–10 weeks old at the beginning of the experiments), which harbours ∼29 tandem copies of a recombinant λgt10 phage vector on each copy of chromosome 3 [Shwed et al., 2010]. The λgt10 vector contains a mutation‐reporting Escherichia coli lacZ gene that can be used to quantify the lacZ MF. Two separate exposure experiments were conducted. For the acute exposures, four groups of Muta™Mouse males (n = 6–8 per group) were given a single acute dose of phosphate buffer (vehicle control) or 25, 50, or 100 mg/kg ENU dissolved in phosphate buffer via oral gavage (volume = 5 μL/g). For the subchronic exposures, another four groups of Muta™Mouse males (n = 6–7 per group) were given repeat doses of phosphate buffer or 1, 2, or 5 mg/(kg day) ENU dissolved in phosphate buffer via oral gavage for 28 consecutive days (thus, total doses were 28, 56, or 140 mg/kg, respectively). For both experiments, mice were euthanized by cervical dislocation under isofluorane anaesthesia 70 days after exposures were completed. The 70‐day period ensures that sperm in the cauda epididymis were all derived from cells that were spermatogonial stem cells throughout the entire exposure period [O'Brien et al., 2014]. After this time, cauda epididymides were collected, flash frozen in liquid nitrogen, and stored at −80°C.
Isolation of Genomic DNA from Cauda Epididymis Sperm
Genomic DNA was isolated from sperm from the cauda epididymides using methods described in [O'Brien et al., 2014]. Briefly, each cauda epididymis was thawed on ice and minced in 1.5 ml of phosphate buffered saline (PBS). The resulting cell suspension was filtered through a steel mesh filter (size 80 mesh, 190 μm pore size). The cells were pelleted by centrifugation and re‐suspended in 1 ml cold 1X saline sodium citrate (SSC). Somatic cells (non‐sperm) were lysed by the addition of 15 µl of 10% sodium dodecyl sulfate (SDS) followed by vigorous shaking for 30 s. The un‐lysed sperm were pelleted by centrifugation and re‐suspended in 940 µl cold 0.2× SSC. For the digestion of sperm, 100 µl 10% SDS, 120 µl β‐mercaptoethanol, 20 µl 0.5 M ethylenediaminetetraacetic acid (EDTA, pH 8), and 20 µl proteinase K (60 mg/ml) were added to the suspension followed by an overnight incubation at 37°C with rotation. High molecular weight genomic DNA was isolated from the sperm digest by multiple phenol/chloroform extractions as described in [O'Brien et al., 2014]. A final extraction using chloroform:isoamyl alchohol (24:1) was performed before DNA was precipitated by the addition of 2 volumes of ethanol (EtOH). The DNA precipitate was spooled, washed in 70% EtOH, re‐dissolved in 40 µl of Tris–EDTA buffer, pH 8, and then stored at 4°C.
LacZ Mutant Frequency
LacZ MF in isolated sperm DNA was determined by a positive selection assay as described in [O'Brien et al., 2014]. Briefly, λgt10 phage vectors were recovered from genomic DNA (∼1–4 µg) using Transpack Packaging Extract kits (Agilent Technologies, Mississauga, ON, Canada) according to the manufacturer's instructions. The packaged phages were used to infect a lacZ−/galE− E. coli host. The infected bacteria were plated on agar plates containing 0.3% phenyl‐β‐d‐galactopyranoside (P‐Gal) to detect mutants, or agar without P‐Gal to determine the total number of plaque‐forming units (pfu). A minimum of 130,000 total pfu were scored for each animal (average = 190,000 pfu. The MF was calculated by dividing the number of mutant plaques by the total pfu count. The induced response was compared with the control group with the glm function in R, using the quasibinomial error distribution to account for overdispersion in the data. The resulting P values were adjusted for multiple comparisons using a Bonferroni correction. The no observable genotoxic effect level (NOGEL) was identified as the highest dose where MF was not significantly greater than control (P > 0.05).
Dose–Response Modeling and Benchmark Dose Analysis
Dose–response modeling was performed using the U.S. Environmental Protection Agency's (EPA) Benchmark Dose Software (BMDS, v2.5) as described in [Johnson et al., 2014]. The linear, quadratic, exponential, and power models were fit to MF data that were transformed by 105. Likelihood ratio tests (LRTs) within BMDS were used to select the most appropriate variance model for our data sets. The fit models were then rated using two metrics: a P value from a goodness‐of‐fit LRT and the Akaike Information Criterion (AIC). The goodness‐of‐fit LRT tests the null hypothesis that the fit model does not significantly differ from a partially saturated model (a model where only the variance parameters are restricted to a non‐constant normal model; the response parameters for each dose group are estimated independently and not by a curve function). A P value > 0.1 indicates a good fit. The AIC is a measure of the relative fit of the model which adjusts for the model's complexity. A lower AIC indicates a better model.
To determine the benchmark dose (BMD), the benchmark response (BMR) was set to one standard deviation of the control group above the estimated response of the model at dose = zero. The BMDL was defined as the lower 95% confidence limit for the model‐extrapolated dose that corresponds to the BMR.
Linearity before NOGEL, Breakpoint Dose, and Slope Transition Dose
We used the “drsmooth” R package to assess several other characteristics of the dose–response relationship [Johnson et al., 2014]. We tested whether the slope of the dose–response before the identified NOGEL was significantly greater than zero. We used the “segmented” tool to identify the dose at which the slope of a fitted bilinear model becomes greater than zero (i.e., the breakpoint dose, BPD). The lower 95% confidence limit for the BPD (BPDL) was also determined. Finally, we used the “smooth” tool to identify the dose at which the slope of a fitted smoothing regression spline first becomes significantly greater than zero (i.e., the slope transition dose, STD), as well as its lower 95% confidence limit (STDL).
RESULTS
Neither exposure regimen produced overt signs of toxicity. Testicular weight, corrected for body weight, was not significantly different from controls in any of the dose groups examined in this study (data not shown). The lacZ MFs in sperm derived from spermatogonial stem cells exposed acutely or subchronically to ENU are summarized in Table 1. Acute ENU exposure resulted in a dose‐dependent increase in MF that was highly significant starting at the lowest dose tested (P = 0.003 at 25 mg/kg). In contrast, MF was less affected when animals were given comparable total doses spread over a 28‐day subchronic exposure. MF did not significantly differ from control levels in the two lowest dose groups. The no observable genotoxic effect level (NOGEL) for the present study was thus 56 mg/kg [or 2 mg/(kg day)]. A significant increase in MF (P = 0.005) after subchronic exposure was only observed in the high dose group of 140 mg/kg total dose [5 mg/(kg day)].
Table 1.
Summary of lacZ Mutant Frequency in Sperm from the Cauda Epididymis of Muta™Mouse Males Exposed Acutely or Subchronically to ENU
| Experiment | Dose (mg/kg) | n | # Plaques | # Mutants | Avg MFa (×105) | SDa (×105) | P value |
|---|---|---|---|---|---|---|---|
| Acute | 0 | 8 | 1,884,624 | 39 | 2.3 | 1.1 | – |
| 25 | 6 | 1,015,702 | 67 | 6.7 | 2.8 | 0.003 | |
| 50 | 8 | 1,296,012 | 134 | 10.7 | 3.8 | <0.001 | |
| 100 | 8 | 1,560,910 | 177 | 11.5 | 4.9 | <0.001 | |
| Subchronic | 0 | 6 | 1,356,479 | 42 | 3.1 | 1.2 | – |
| 28 | 7 | 1,664,619 | 67 | 4.0 | 1.4 | 0.595 | |
| 56 | 6 | 1,439,974 | 55 | 3.9 | 1.3 | 0.958 | |
| 140 | 7 | 1,346,538 | 128 | 9.5 | 3.9 | 0.005 |
Average mutant frequency (Avg MF) and standard deviation (SD) are based on the arithmetic mean for individual animals.
Dose–response data were fit to linear, quadratic, exponential or power models using US‐EPA's BMDS. The variance LRT within BMDS indicated that a non‐constant normal variance model was most appropriate for our data sets. The model fitting results are shown in Figure 1. The linear model exhibited poor goodness‐of‐fit at P < 0.1 for both the acute and subchronic data. Both the goodness‐of‐fit LRT P values and the AIC value indicated that increasing the complexity compared to the linear model improved fit, suggesting that the dose–response for both data sets was nonlinear. Based on the fit metrics (P = 0.6305, AIC = 97.3), the quadratic model yielded the best fit for the acute data. Although the low dose region of the curve appeared linear, a quadratric term was required to fit the high dose, where the mutational effect seemed to saturate. In fact, when the high dose group was removed from the data set, the linear model provided the best fit (data not shown). For the subchronic data, both the exponential and power models provided the best fit with comparable scores, with the exponential model having a slightly higher P value and lower AIC.
Figure 1.
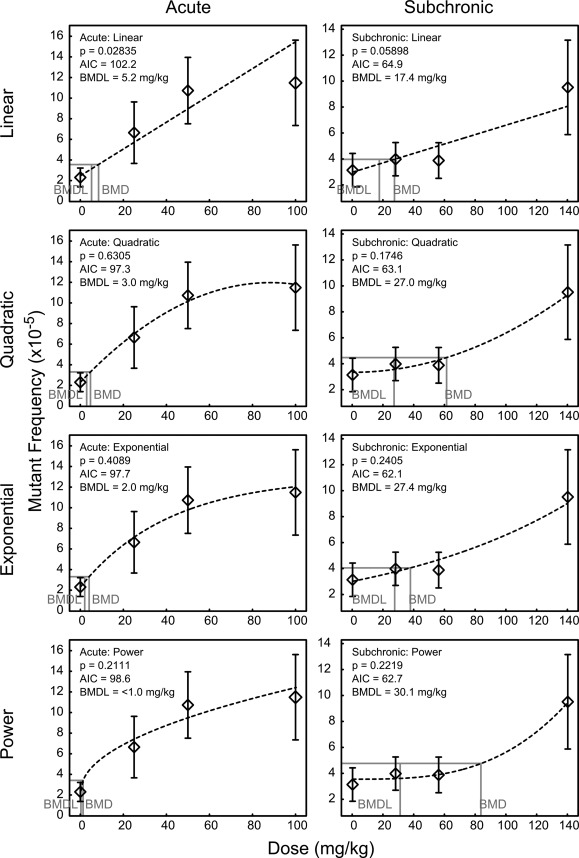
Dose–response modeling of the ENU‐induced lacZ mutant frequency following acute exposure or a 28‐day subchronic exposure. Linear, quadratic, exponential and power models are shown for both data sets. Models fit to the acute data are shown in the left column and models fit the subchronic data are shown on the right. The goodness‐of‐fit likelihood ratio test P values and Akaike Information Criterion (AIC) scores are shown for each model, as are the extrapolated benchmark doses (BMD) and their associated lower 95% confidence limits (BMDL). P > 0.1 indicates a good fit. A lower AIC indicates a better model. Diamond shapes represent the arithmetic mean mutant frequency from each dose group. Error bars represent the 95% confidence interval of the mean.
BMDLs were determined by setting the BMR to one standard deviation above the model‐estimated control MF. Both the acute and subchronic experiments had comparable standard deviation in their respective control groups (SD = ±1.1 and 1.2 × 10−5, respectively). The BMDL from the best fitting model for the acute exposures (quadratic model) was 3.0 mg/kg ENU. The best‐fitting model for the subchronic data was the exponential model, which yielded a BMDL of 27.4 mg/kg total dose [or ∼1.0 mg/(kg day)]. The power model, which had comparable fit scores for the subchronic data, yielded a BMDL of 30.1 mg/kg [or 1.1 mg/(kg day)]. These results show that the BMDLs for acute and chronic exposures differ by a factor of approximately 10‐fold.
Next, we tested whether the slope of the dose–response up to the NOGEL was significantly different from zero. A NOGEL was not identified in the present study for the acute exposures. The NOGEL for the subchronic exposures was 56 mg/kg [2 mg/(kg day)]. The slope of the subchronic dose–response up to and including the NOGEL was not significantly different than zero (P = 0.335).
Breakpoint dose (BPD) and slope‐transition dose (STD) were determined for both the acute and subchronic data sets using the “drsmooth” toolset. These results and the resulting bilinear curves and smoothing regression splines for each data set are shown in Figure 2. The lower confidence limits for the BPD and STD (BPDL and STDL, respectively) were both below zero for the acute data set, indicating that both of these results were not significant. In contrast, both BPDL and STDL were above zero for the subchronic dose–response, indicating a significant BPD and STD. The BPD for the subchronic dose–response was 51.7 mg/kg (BPDL = 22.7 mg/kg) and the STD was 29.7 mg/kg (STDL = 18.6 mg/kg). Although the BPD tool in the drsmooth package is designed for data with homogeneous normal variance, it is not greatly influenced by deviations from this type of distribution [Johnson et al., 2014]. Nevertheless, since our data had non‐constant variance and did not meet this requirement, we performed a log transformation to the MF data to achieve a normal homogeneous variance, as determined by Shapiro–Wilk and Bartlett tests. When BPD was determined using the transformed data set, the results were consistent with the results from the untransformed data set (data not shown). Collectively, the results show that acute and subchronic exposure to similar total doses yielded different dose–response curves for lacZ MF.
Figure 2.
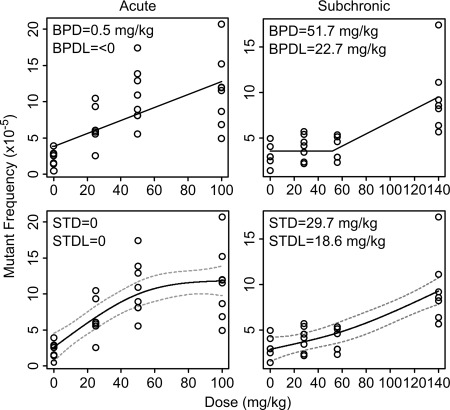
Breakpoint dose (BPD) and slope transition dose (STD) modeling of the ENU‐induced lacZ mutant frequency following acute exposure or a 28‐day subchronic exposure. The bilinear models to derive BPD are shown in the top row. The smoothing regression spline models to determine STD are shown in the bottom row. Models for the acute data are shown in the left column and models fit the subchronic data are shown on the right. The BPD, STD, and their respective lower 95% confidence limits (BPDL and STDL) are shown for each model. Circles represent the mutant frequency of individual animals. The dotted lines in the STD models represent the 95% confidence interval of the estimated dose–response function.
DISCUSSION
We studied the dose–response relationship of spermatogonial stem cells exposed acutely or subchronically to ENU. Our results show that acute exposures of stem cell spermatogonia to high doses of ENU caused a dose‐dependent increase in lacZ MF that was significant at all doses tested. When similar total doses were fractionated over a 28‐day subchronic exposure, lacZ MF was indistinguishable from background levels, for doses up to 2 mg/(kg day). This lack of response in MF to accumlative ENU exposure in spermatogonial stem cells is suggestive of a saturable repair response, where at lower doses the chemically‐induced DNA damage is sufficiently repaired, but overwhelms the DNA repair capacity as the dose increases [Julien et al., 2009].
Model fitting further accentuated the difference in the lacZ mutation response between acute and subchronic exposures. The acute dose–response was best described by a quadratric model that was linear in the low dose region. A quadratic term was required to accommodate the high dose, where mutational effects were saturated. This saturation is presumably due to cytotoxicity in stem cells that bear too great a mutational burden in the high dose and are not able to progress through spermatogenesis. On the other hand, the response to subchronic exposure was best described by the exponential and power models. Both of these models feature a sublinear response in the low dose region of the curve. A possible biological interpretation of the exponential and power‐type response is that there may be no dose at which mutational effects do not occur. However, it has been proposed that in the case of the power response or similar models, a so called “practical threshold” be invoked in the low dose region, where predicted effects are indistinguishable from the background variance due to practical and technical limitations in mutation detection [Lutz, 1998]. In fact, when the shape of the dose–response for subchronic exposures was assessed below doses that caused significant effects (i.e., up to the NOGEL) the slope was statistically indistinguishable from zero.
The exponential model, which had the best fit metrics when considering all subchronic doses, was less flat in the low dose region and had less curvature than the power model. However, it is likely that the inclusion of more dose groups in the low dose region of the curve would favor a “flatter” exponential type model or favor the power model. Thus, although a higher resolution in the low dose range is required to fully characterize the precise shape of the dose–response curve, our results clearly indicate that the dose–response for lacZ mutation in spermatogonial stem cells exposed to ENU is sublinear for low dose subchronic exposure, with a NOGEL of 2 mg/(kg day). This result is highly supportive of a saturable repair process in spermatogonial stem cells. The evidence is further corroborated when considering that a significant BPD and STD were identified for the subchronic data. These two metrics describe the dose at which the slope of the dose–response transitions significantly from zero. The fact that both BPD and STD were significantly above zero further suggests that the low‐dose region of the dose–response curve does not increase linearly with dose.
A sublinear response to the mutagenicity of ENU in spermatogonial stem cells is supported by other studies using both traditional and modern germ cell mutation assays. Both Russell et al. [1982] and Favor et al. [1990] reported a threshold‐type response in mutant offspring following acute ENU exposure to spermatogonia stem cells using the specific locus test (SLT). The threshold dose for these two studies was estimated at 39 and 34 mg/kg, respectively. Although these studies are in support of a sublinear effect, the detection of a threshold at acute doses above 25 mg/kg conflicts with the findings of the present study. Our results showed a linear response using similar acute doses. However, the SLT data had relatively small sample sizes in the low dose groups (25 and 40 mg/kg) relative to the background MF. For example, the Russell et al. study examined less than 4000 offspring in the 25 mg/kg group and observed zero mutants. This may not have provided enough sensitivity to detect mutations in the low dose groups; thus, lack of power in the SLT studies weakens their conclusion of a threshold effect after acute exposure. In contrast, the TGR assay employed in the present study provided excellent sensitivity, where an average of ∼6 lacZ mutants was observed for each control animal, and highly significant effects were detected after acute exposure to 25 mg/kg ENU. This increased sensitivity is a result of the larger number of sperm sampled in the TGR assay compared to the SLT. The difference in the shape of the response curve between the SLT studies and the present study (i.e., high threshold dose for SLT study) may also be attributed to inter‐strain differences in ENU sensitivity [Justice et al., 2000] (Muta™Mouse, derived from a DBA2 and BALB/c cross, in the present study versus 101/Rl × C3H/Rl crosses in SLT study) or differences in exposure routes (intraperitoneal injection in the SLT study versus gavage in the present study). In fact, an apparent sublinear response in the lacZ MF of Muta™Mouse spermatogonia stem cells acutely exposed to ENU via intraperitoneal injection was previously reported [van Delft and Baan, 1995]. In that study, although the sublinearity was not confirmed by a model fitting analysis, no significant effects were detected after acute intraperitoneal exposure up to 50 mg/kg. The lower genotoxic response observed in the SLT and in lacZ MF following intraperitoneal injection compared to the present study suggests that the saturation dose in spermatogonial stem cells may be dependent on the route of exposure.
The presence of a sublinear genotoxic effect after subchronic exposure to alkylating agents is also supported by results for multiple genotoxicity endpoints (including lacZ mutation) in several somatic tissues. Several points of departure were estimated for lacZ MF in the small intestine and spleen following acute exposure to ENU [van Delft et al., 1998; Johnson et al., 2014]. Acute NOGELs of 10 and 25 mg/kg were reported for the small intestine and spleen respectively. In the present study, we were able to detect significant effects in spermatogonia at 25 mg/kg, implying the germline might be more susceptible to ENU mutagenicity. However, as noted in [Johnson et al., 2014], NOGEL and bilinear‐model‐derived metrics such as BPD are very sensitive to dose spacing and data quality, and that BMDL is a more robust metric that is more suitable for interstudy comparisons. We thus determined BMDLs using the same approach as in [Johnson et al., 2014]. The acute BMDL for lacZ MF in spermatogonial stem cells (based on the quadratic model) from the present study (2.9 mg/kg) was more comparable, albeit still lower, than in small intestine or spleen (5.5 and 11.7 mg/kg, respectively). The BMDL for subchronic exposure was higher (27.4 mg/kg total dose, based on the exponential model), again demonstrating less sensitivity to fractionated exposure. No study, to our knowledge has determined subchronic BMDLs for ENU in other tissues for comparison. One study reported that fractionated exposure to the alkylating agent EMS resulted in a sublinear response in several somatic tissues at doses that would normally induce a linear response when given acutely [Gocke and Muller, 2009; Gollapudi et al., 2013]. The EMS study also included ENU exposures but the doses administered were too genotoxic to detect a sublinear effect. However, based on their adduct data and results from a drinking water study [Cosentino and Heddle, 1999], the authors suggested that a point of departure for ENU mutation in somatic tissue is very likely at subchronic exposures below 1 mg/(kg day). In contrast to the acute data, these observations imply the germline is less sensitive to ENU mutagenesis. However, with both the acute and subchronic data, several confounding factors such as exposure route and strain variability make it difficult to make reliable comparisons between tissues.
Given the observed sublinear responses to EMS and ENU in somatic tissues, and the sublinear response observed for ENU in spermatogonial stem cells in the present study, it is likely that both somatic cells and germ cells have similar mechanisms responsible for a sublinear response to alkylating agents. The proposed mechanism for the observed sublinear response involves the saturable repair of alkyl DNA adducts by DNA alkyltransferases and nucleotide excision repair [Bronstein et al., 1991, 1992]. The most mutagenic ENU‐induced DNA adducts appear to be O4‐ethyl thymine, O2‐ethyl thymine, and O6‐ethyl guanine [Noveroske et al., 2000]. This is supported by the fact that ENU‐induced mutation spectra tend to be enriched for AT–TA transversions, AT–GC transitions, and GC–AT transitions [Douglas et al., 1995; Noveroske et al., 2000]. A saturable repair mechanism, at least for O6‐ethyl guanine, is further supported by a sublinear response in O6‐adduct formation following ENU exposure [van Zeeland et al., 1990] and likely involves alkylguanine‐DNA alkyltransferase [Pegg, 2011; Thomas et al., 2013]. The mechanisms involved in the repair of the O4‐ and O2‐adducts appear to be less efficient than the O6‐adduct repair, and have yet to be precisely identified. There is evidence that baseline genetic integrity is superior in the germline compared to somatic cells [Murphey et al., 2013], suggesting possible differences in the basal activity or possibly even in the mechanisms of repair machinery between these two cell types. This is supported by the relatively high NOGEL and BMDL observed for subchronically exposed spermatogonial stem cells, although as previously mentioned, more data in other tissues using similar experimental designs is required to make a more confident comparison.
The detoxification of ENU by glutathione and other metabolic enzymes may also play a critical role in varying the rate of exposure in the testes [Wilhelm et al., 1997] thus affecting the different observed dose–responses observed after acute vs subchronic exposures. However, in the absence of experimental data on tissue concentration of the compound of interest, its most active metabolite, or the DNA adduct frequency it is not possible to assess the respective roles of detoxification and DNA repair in the saturation of MF at high dose (for acute exposure) and the lack of induction of MF at low doses (for subchronic exposure). Physiologically based pharmacokinetic (PBPK) modelling or comparison of the acute and subchronic dose–response in repair‐deficient strains would also help clarify this issue.
There is increasing support for employing quantitative dose–response modeling of genetic toxicology data for the risk assessment of DNA‐reactive carcinogens. To date, the use of genetic toxicology data has been limited almost entirely to qualitative identification of hazard. Furthermore, low‐dose risk based on other toxic endpoints for genotoxic carcinogens is generally extrapolated adopting a linear dose–response approach. However, accumulating evidence for a sublinear genotoxic response has led the regulatory community to reconsider its approach for evaluating regulatory exposure limits for these compounds. The International Life Science Institute (ILSI) and the International Workshop on Genotoxicty Testing (IWGT) are currently investigating the potential use of dose–response data for establishing a genotoxic point of departure (e.g., NOGEL, BPD, STD, or BMDL) that can be applied in a similar fashion as the no observable adverse effect level (NOAEL) which is typically used for determining exposure limits for non‐DNA reactive toxic agents [Johnson et al., 2009; Pottenger and Gollapudi, 2010; Gollapudi et al., 2013]. As the TGR mutation assay is expected to be increasingly applied in genotoxicity test batteries following the recent release of the TGR OECD test guideline [OECD, [Link]], it is important to test the feasibility of its adoption in future testing approaches for germ cells. Our study demonstrates the TGR assay as an effective and economical means for identifying points of departure using dose–response modeling. Additionally, our results indicate that a fractionated low dose regimen can be used to identify a point of departure that would otherwise be missed or underestimated if equal total doses were given acutely. This is not to say that the point at which DNA repair becomes saturated cannot be observed using an acute exposure design. Theoretically, a point of departure can be identified if sufficiently low acute doses are used. Our findings do show that a completely linear dose–response may be indicative that lower or fractionated doses should be considered to fully characterize the dose–response.
In conclusion, we have demonstrated that the dose–response for lacZ mutation in spermatogonia stem cells exposed to ENU is sublinear for low dose 28‐day subchronic exposure. The dose–response was best described by the sublinear exponential and power models. These models may be used to determine a point of departure dose at which no significant genotoxic effects are detected over the measured background. In the present study we identified a NOGEL dose of 2 mg/(kg day), where the MF in exposed animals was not significantly different from controls. The same total dose administered acutely elicited a dose dependent response that was significant at all doses. These results are suggestive of a saturable repair mechanism. Finally, we also demonstrate the TGR mutation assay as an effective modern method for assessing dose–response relationships in germ cells. A 28‐day subchronic low dose exposure regimen is recommended to capture low dose effects that would otherwise be missed by an acute exposure design, although theoretically both regimens can be used to identify a point of departure dose. This assay may prove especially efficient should an integrated approach for simultaneous somatic and germ cell testing be achieved.
AUTHOR CONTRIBUTIONS
J.M. O'Brien was responsible for study design, data collection, data analysis, and manuscript preparation. M. Walker and A. Sivathayalan contributed to the development of the dose–response modeling approach and the interpretation of the modeling results. G.R. Douglas, C.L. Yauk, and F. Marchetti secured the funding for the study and were responsible for study conception, study design and manuscript revisions. All authors approved the final manuscript. All authors declare that there are no conflicts of interest.
ACKNOWLEDGMENTS
This research was funded by the Canadian Regulatory System for Biotechnology (CRSB) and Chemicals Management Plan (CMP) initiatives.
REFERENCES
- Akaike H. 1974. A new look at the statistical model identification. IEEE Trans Autom Control 19:716–723. [Google Scholar]
- Bolker B. 2013. bbmle: Tools for general maximum likelihood estimation. http://cran.r-project.org/web/packages/bbmle/bbmle.pdf (accessed July 3, 2014).
- Bronstein SM, Cochrane JE, Craft TR, Swenberg JA, Skopek TR. 1991. Toxicity, mutagenicity, and mutational spectra of N‐ethyl‐N‐nitrosourea in human cell lines with different DNA repair phenotypes. Cancer Res 51:5188–5197. [PubMed] [Google Scholar]
- Bronstein SM, Skopek TR, Swenberg JA. 1992. Efficient repair of O6‐ethylguanine, but not O4‐ethylthymine or O2‐ethylthymine, is dependent upon O6‐alkylguanine‐DNA alkyltransferase and nucleotide excision repair activities in human cells. Cancer Res 52:2008–2011. [PubMed] [Google Scholar]
- Bryce SM, Avlasevich SL, Bemis JC, Phonethepswath S, Dertinger SD. 2010. Miniaturized flow cytometric in vitro micronucleus assay represents an efficient tool for comprehensively characterizing genotoxicity dose–response relationships. Mutat Res 703:191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Mittelstaedt RA, Pearce MG, Allen BC, Soeteman‐Hernández LG, Johnson GE, Bigger CA, Heflich RH. 2014. Quantitative dose–response analysis of ethyl methanesulfonate genotoxicity in adult gpt‐delta transgenic mice. Environ Mol Mutagen 55:385–399. [DOI] [PubMed] [Google Scholar]
- Conrad DF, Keebler JE, DePristo MA, Lindsay SJ, Zhang Y, Casals F, Idaghdour Y, Hartl CL, Torroja C, Garimella KV, et al. 2011. Variation in genome‐wide mutation rates within and between human families. Nat Genet 43:712–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosentino L, Heddle JA. 1999. Effects of extended chronic exposures on endogenous and transgenic loci: Implications for low‐dose extrapolations. Environ Mol Mutagen 34:208–215. [PubMed] [Google Scholar]
- Demarini DM. 2012. Declaring the existence of human germ‐cell mutagens. Environ Mol Mutagen 53:166–172. [DOI] [PubMed] [Google Scholar]
- Doak SH, Jenkins GJ, Johnson GE, Quick E, Parry EM, Parry JM. 2007. Mechanistic influences for mutation induction curves after exposure to DNA‐reactive carcinogens. Cancer Res 67:3904–3911. [DOI] [PubMed] [Google Scholar]
- Douglas GR, Jiao J, Gingerich JD, Gossen JA, Soper LM. 1995. Temporal and molecular characteristics of mutations induced by ethylnitrosourea in germ cells isolated from seminiferous tubules and in spermatozoa of lacZ transgenic mice. Proc Natl Acad Sci USA 92:7485–7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favor J, Sund M, Neuhauser‐Klaus A, Ehling UH. 1990. A dose–response analysis of ethylnitrosourea‐induced recessive specific‐locus mutations in treated spermatogonia of the mouse. Mutat Res 231:47–54. [DOI] [PubMed] [Google Scholar]
- Gocke E, Muller L. 2009. In vivo studies in the mouse to define a threshold for the genotoxicity of EMS and ENU. Mutat Res 678:101–107. [DOI] [PubMed] [Google Scholar]
- Gollapudi BB, Johnson GE, Hernandez LG, Pottenger LH, Dearfield KL, Jeffrey AM, Julien E, Kim JH, Lovell DP, Macgregor JT, et al. 2013. Quantitative approaches for assessing dose–response relationships in genetic toxicology studies. Environ Mol Mutagen 54:8–18. [DOI] [PubMed] [Google Scholar]
- Johnson GE, Doak SH, Griffiths SM, Quick EL, Skibinski DO, Zair ZM, Jenkins GJ. 2009. Non‐linear dose–response of DNA‐reactive genotoxins: Recommendations for data analysis. Mutat Res 678:95–100. [DOI] [PubMed] [Google Scholar]
- Johnson GE, Zair Z, Bodger OG, Lewis PD, Rees BJ, Verma JR, Thomas AD, Doak SH, Jenkins GJS. 2012. Investigating mechanisms for non‐linear genotoxic responses, and analysing their effects in binary combination. Genes Environ 34:179–185. [Google Scholar]
- Johnson GE, Soeteman‐Hernandez LG, Gollapudi BB, Bodger OG, Dearfield KL, Heflich RH, Hixon JG, Lovell DP, Macgregor JT, Pottenger LH, et al. 2014. Derivation of point of departure (PoD) estimates in genetic toxicology studies and their potential applications in risk assessment. Environ Mol Mutagen 55:609–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julien E, Boobis AR, Olin SS, Ilsi Research Foundation Threshold Working Group . 2009. The key events dose–response framework: A cross‐disciplinary mode‐of‐action based approach to examining dose–response and thresholds. Crit Rev Food Sci Nutr 49:682–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice MJ, Carpenter DA, Favor J, Neuhauser‐Klaus A, Hrabe de Angelis M, Soewarto D, Moser A, Cordes S, Miller D, et al. 2000. Effects of ENU dosage on mouse strains. Mamm Genome 11:484–488. [DOI] [PubMed] [Google Scholar]
- Kong A, Frigge ML, Masson G, Besenbacher S, Sulem P, Magnusson G, Gudjonsson SA, Sigurdsson A, Jonasdottir A, Jonasdottir A, et al. 2012. Rate of de novo mutations and the importance of father's age to disease risk. Nature 488:471–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz WK. 1998. Dose–response relationships in chemical carcinogenesis: Superposition of different mechanisms of action, resulting in linear‐nonlinear curves, practical thresholds, J‐shapes. Mutat Res 405:117–124. [DOI] [PubMed] [Google Scholar]
- Lynch AM, Giddings A, Custer L, Gleason C, Henwood A, Aylott M, Kenny J. 2011. International Pig—A gene mutation assay trial (stage III): Results with N‐methyl‐N‐nitrosourea. Environ Mol Mutagen 52:699–710. [DOI] [PubMed] [Google Scholar]
- Muller L, Gocke E, Lave T, Pfister T. 2009. Ethyl methanesulfonate toxicity in Viracept—A comprehensive human risk assessment based on threshold data for genotoxicity. Toxicol Lett 190:317–329. [DOI] [PubMed] [Google Scholar]
- Murphey P, McLean DJ, McMahan CA, Walter CA, McCarrey JR. 2013. Enhanced genetic integrity in mouse germ cells. Biol Reprod 88:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noveroske JK, Weber JS, Justice MJ. 2000. The mutagenic action of N‐ethyl‐N‐nitrosourea in the mouse. Mamm Genome 11:478–483. [DOI] [PubMed] [Google Scholar]
- O'Brien JM, Beal MA, Gingerich JD, Soper L, Douglas GR, Yauk CL, Marchetti F. 2014. Transgenic rodent assay for quantifying male germ cell mutation frequency. J Vis Exp 90:e51576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OECD. 2009. Detailed review paper on transgenic rodent assays. Organization for Economic Co‐operation and Development. http://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=ENV/JM/MONO%282009%297&docLanguage=En (accessed July 3, 2014).
- OECD. 2013. OECD guideline for the testing of chemicals: Transgenic rodent somatic and germ cell gene mutation assays. doi: 10.1787/9789264203907-en. [DOI]
- Pegg AE. 2011. Multifaceted roles of alkyltransferase and related proteins in DNA repair, DNA damage, resistance to chemotherapy, and research tools. Chem Res Toxicol 24:618–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pottenger LH, Gollapudi BB. 2010. Genotoxicity testing: Moving beyond qualitative “screen and bin” approach towards characterization of dose–response and thresholds. Environ Mol Mutagen 51:792–799. [DOI] [PubMed] [Google Scholar]
- Pottenger LH, Schisler MR, Zhang F, Bartels MJ, Fontaine DD, McFadden LG, Bhaskar Gollapudi B. 2009. Dose–response and operational thresholds/NOAELs for in vitro mutagenic effects from DNA‐reactive mutagens, MMS and MNU. Mutat Res 678:138–147. [DOI] [PubMed] [Google Scholar]
- Russell WL, Hunsicker PR, Raymer GD, Steele MH, Stelzner KF, Thompson HM. 1982. Dose–response curve for ethylnitrosourea‐induced specific‐locus mutations in mouse spermatogonia. Proc Natl Acad Sci USA 79:3589–3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAS Institute Inc. 2008. SAS/STAT 9.2 User's Guide. Cary, NC, USA: SAS Institute Inc. [Google Scholar]
- Shwed PS, Crosthwait J, Douglas GR, Seligy VL. 2010. Characterisation of MutaMouse lambdagt10‐lacZ transgene: Evidence for in vivo rearrangements. Mutagenesis 25:609–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas AD, Jenkins GJ, Kaina B, Bodger OG, Tomaszowski KH, Lewis PD, Doak SH, Johnson GE. 2013. Influence of DNA repair on nonlinear dose–responses for mutation. Toxicol Sci 132:87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Delft JH, Baan RA. 1995. Germ cell mutagenesis in lambda lacZ transgenic mice treated with ethylnitrosourea; comparison with specific‐locus test. Mutagenesis 10:209–214. [DOI] [PubMed] [Google Scholar]
- van Delft JH, Bergmans A, van Dam FJ, Tates AD, Howard L, Winton DJ, Baan RA. 1998. Gene‐mutation assays in lambda lacZ transgenic mice: Comparison of lacZ with endogenous genes in splenocytes and small intestinal epithelium. Mutat Res 415:85–96. [DOI] [PubMed] [Google Scholar]
- van Zeeland AA, de Groot A, Neuhauser‐Klaus A. 1990. DNA adduct formation in mouse testis by ethylating agents: A comparison with germ‐cell mutagenesis. Mutat Res 231:55–62. [DOI] [PubMed] [Google Scholar]
- Wilhelm D, Bender K, Knebel A, Angel P. 1997. The level of intracellular glutathione is a key regulator for the induction of stress‐activated signal transduction pathways including Jun N‐terminal protein kinases and p38 kinase by alkylating agents. Mol Cell Biol 17:4792–4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zair ZM, Jenkins GJ, Doak SH, Singh R, Brown K, Johnson GE. 2011. N‐methylpurine DNA glycosylase plays a pivotal role in the threshold response of ethyl methanesulfonate‐induced chromosome damage. Toxicol Sci 119:346–358. [DOI] [PubMed] [Google Scholar]


