Figure 1.
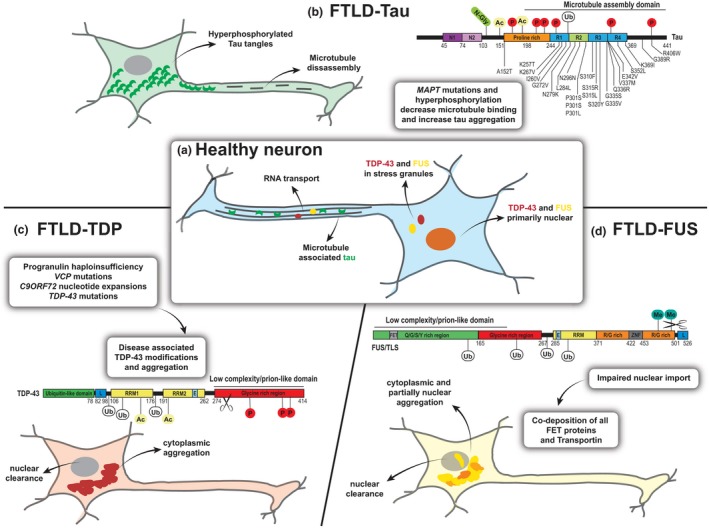
The three main types of FTD pathology are characterized by the accumulation of distinct protein aggregates. (a) In healthy neurons, tau (green crescent) is mainly distributed in axons and regulates microtubule stability. TDP‐43 (red) and FUS (yellow) are primarily found in the nucleus, where they are involved in several steps of RNA metabolism. In the cytoplasm, both proteins participate in dynamic RNA granules, facilitating axonal transport or forming as part of the cell stress response. (b) In pathological conditions of FTLD‐tau, the protein is dissociated from microtubules and accumulates into pathogenic tangles in the cytoplasm. Mutations in the microtubule‐binding region of the microtubule‐associated protein tau (MAPT) gene are found FTLD‐17 MAPT and can lead to splicing changes and decreased microtubule‐binding affinity. Hyperphosphorylation (P) and other post‐translational modifications (N‐Glyc (N‑glycosylation), Ac (Acetylation), Ub (Ubiquitination) can further enhance the detachment, resulting in microtubule disassembly and the formation of tau aggregates in the cytoplasm (example modification sites are shown). (c) In FTLD‐TDP‐43 several underlying genetic causes (GRN, VCP, TDP‐43, C9ORF72 mutations) and sporadic cases are linked by a common TDP‐43 pathology. TDP‐43 undergoes several disease‐associated modifications including phosphorylation, ubiquitination and C‐terminal cleavage and forms pathogenic inclusions in the cytoplasm leading to a redistribution of the protein, associated with nuclear clearance. (d) Although called FTLD‐FUS, all FET proteins have now been found to co‐aggregate in ubiquitinated inclusions in severe cases of sporadic FTD. Factors impairing the nuclear import of these proteins might lead to cytoplasmic accumulation and following aggregation because of strongly aggregation‐prone low complexity domains. C9ORF72: chromosome 9 open reading frame 72, E: Nuclear export signal, FET: FUS, TAF‐15, EWS protein interacting domain, FTD: frontotemporal dementia, FTLD: frontotemporal lobar degeneration, FUS: fused in sarcoma, L: nuclear localization signal, N1, N2: near‐amino‐terminal inserts, Q/G/S/Y‐rich: glutamine‐glycine‐serine‐tyrosine rich region, R1, R2, R3, R4: carboxy‐terminal repeat domain, R/G‐rich: arginine/glycine rich region, RRM: RNA recognition motif, TDP‐43: TAR DNA‐binding protein 43, VCP: valosin‐containing protein, ZNF: zinc finger domain.
