Abstract
Aim
Rwanda has invested heavily in improving maternal and child health, but knowledge is limited regarding social equity in perinatal survival. We analysed whether perinatal mortality risks differed between social groups in hospitals in the country's capital.
Methods
A case–control study was carried out on singleton births aged at least 22 weeks of gestation and born in district or tertiary referral hospitals in Kigali from July 2013 to May 2014. Perinatal deaths were recorded as they occurred, with the next two surviving neonates born in the same hospital selected as controls. Conditional logistic regression was used to determine social determinants of perinatal death after adjustments for potential confounders.
Results
We analysed 234 perinatal deaths and 468 controls. Rural residence was linked to an increased risk of perinatal death (OR = 3.31, 95% CI 1.43–7.61), but maternal education or household asset score levels were not. Having no health insurance (OR = 2.11, 95% CI 0.91–4.89) was associated with an increased risk of perinatal death, compared to having community health insurance.
Conclusion
Living in a rural area and having no health insurance were associated with an increased risk of perinatal mortality rates in the Rwandan capital, but maternal education and household assets were not.
Keywords: Perinatal mortality, Rwanda, Social differentials, Urban hospital
Key notes.
Following heavy investment in Rwanda's maternal and child health services, we analysed whether perinatal mortality risks differed between social groups in hospitals in the country's capital.
Our study of 234 perinatal deaths and 468 controls showed that living in a rural area or having no health insurance increased the risk of perinatal death.
Levels of maternal education or household wealth were not associated with perinatal mortality risks.
Introduction
Approximately 4.8 million perinatal deaths are recorded annually throughout the world 1, 2. These deaths include 2.6 million stillbirths and 2.2 million early neonatal deaths occurring during the perinatal period, which extends from 22 weeks of gestation up to the first week of life. Perinatal mortality, which is defined as the sum of stillbirths and early neonatal deaths per 1000 births, constitutes a major public health issue, particularly in low‐ and middle‐income countries where 98% of perinatal deaths occur 3. The disparity in perinatal survival between low‐ and high‐income countries suggests that most perinatal deaths could be prevented. Reducing these deaths could also contribute to improving neonatal survival, as early neonatal deaths constitute 75% of all neonatal deaths 3, 4.
An increasing body of evidence indicates that perinatal mortality differs not only between countries but also within countries 3. Mortality levels are usually lower in households with higher socio‐economic status, which often takes into account parental education or literacy, occupation, place of residence, ethnic or religious affiliations, household wealth or income and possession of health insurance 5, 6, 7. However, the variability of these inequalities over time suggests that they could be affected by policy changes, such as the combination of optimal coverage in health services and targeted interventions for socially disadvantaged groups 8. Therefore, there is a need to address social differentials in perinatal survival as they could hamper efforts to improve public health and the development of a healthier society.
In Rwanda, estimates indicated a perinatal mortality rate of 44 deaths per 1000 births in 2005, but these were unequally distributed across different social strata 9.These data were based on national averages, which are used to monitor general trends of perinatal mortality. However, information related to perinatal survival at these facilities is lacking, despite the fact that 69% of women in Rwanda deliver their babies at health facilities 10.
From the late 1990s onwards, the Rwandan government has strengthened the health system as suggested by the World Health Organization (WHO) 11. The WHO's initiative to promote safe motherhood and child health became a priority for national reproductive health policy 12.A national roadmap accelerating the reduction of maternal and newborn mortality was developed and built following recommendations by the Making Pregnancy Safer strategy, which was launched by the WHO and its partners.
A community health insurance scheme, known as Mutuelle de santé, was scaled up to improve access to healthcare services, with particular attention given to poor and other vulnerable people 13. Other insurance regimes were also developed. These included a state insurance scheme called RAMA, which mainly covered civil servants and their dependents, a military medical insurance scheme covering the military and their dependents, and private insurance regimes provided by private institutions or commercial companies 13. In 2010, 78% of households across the country had some form of health insurance and 98% of these were covered by the Mutuelle de santé community health insurance 10.Various interventions were implemented to reduce health inequalities 14, 15.
However, there is still little information about the differentials in perinatal survival rates related to residence, wealth and the educational level of the mothers. This is needed to guide effective interventions. The aim of this study was to analyse social differentials in perinatal mortality in urban hospitals in Kigali, which is the capital of Rwanda.
Subjects and Methods
This was a case–control study, matched for hospital and day of birth, which was carried out at one district hospital and one tertiary referral hospital in the Rwandan capital Kigali, during a 10‐month period from 18 July 2012 to 8 May 2013. The district hospital offers maternal, neonatal and child healthcare services, which constitute the bulk of its clinical activities. Maternal services include antenatal, delivery and postnatal care. In 2012, the catchment area of the district hospital included 10 health centres that covered a population of almost 284 860 inhabitants. The tertiary referral hospital is the largest referral and university hospital in the country, receiving pregnant women who mainly live in the capital. Some of them are referred from rural or urban hospitals or private clinics. In 2012, 7949 births were attended by registered nurses, midwives and doctors in the district hospital and 2150 births in the tertiary referral hospital. The district hospital handled the highest number of deliveries in the country. In that year, the country had a total of 41 district hospitals, which handled 98 395 births, and five tertiary referral hospitals, which handled 4449 deliveries.
Community health insurance was commonly used by patients in both hospitals and state, and military and private insurance policies were also reported. Most of the beneficiaries of these insurance policies were expected to contribute to payments of billable healthcare services received at each visit or hospitalisation, and these were referred to as copayments. The rates of copayments were fixed at 15% for the state and military insurance policies and 10% for the community health insurance. For private insurance, the rates of copayments varied according to the contracts with each insurer, but some of these covered 100% of the costs of necessary care. Copayments were waived for the indigent and poorest people, orphans and needy genocide survivors who were covered by the community health insurance. Uninsured patients were expected to pay the whole cost of their care. This also applied to people using the community health insurance who bypassed referral steps, except emergency cases. The referral policy stipulated that patients should first be seen in public health centres or health posts before being referred to district hospitals and that they, in turn, could refer these patients to the tertiary referral hospitals.
Study participants
We included stillbirths, early neonatal deaths and neonates who survived the first 7 days after birth or until discharge. A stillbirth was defined as a foetal death occurring from 22 completed weeks of pregnancy or weighing at least 500 g at birth. An early neonatal death was considered as a death in the first week following a live birth with a gestational age of at least 22 completed weeks or a birthweight of at least 500 g 3.
Stillbirths and early neonatal deaths were recorded throughout the study period. Controls included preterm or term singleton births who survived the early neonatal period or until discharge. The two surviving neonates born immediately after each perinatal death at the same hospital were selected as controls. We excluded all neonates born elsewhere, those who were seriously ill and twin births. Newborn babies were considered to be seriously ill if they presented with severe infections or intrapartum‐related asphyxia, complications of prematurity or other life‐threatening neonatal illnesses. Such babies usually required close management in neonatal intensive care units.
Data collection
One nurse and one midwife per hospital prospectively collected data using a predesigned semi‐structured questionnaire. These field workers underwent 2 days of training on study procedures and data collection. One doctor recruited from each hospital received the same training and assisted the main investigator to oversee the data collection. After training, a pilot study was conducted for a month before the study started, to validate and standardise the questionnaires and to allow field workers to get familiar with data collection.
Data collectors interviewed women whose babies were enrolled in the study, on their socio‐economic and demographic backgrounds, obstetric history and whether they had health insurance during their pregnancy. They were also asked to report some household assets and facilities. Interviews were conducted with partners or relatives when mothers were not available. Data collectors also gathered relevant information from registry books and medical records in the maternity admission rooms, delivery rooms, maternity operation theatres, maternity wards or neonatal units. Information was also sought from health workers involved in managing the mothers or their babies.
The quality of data was controlled by repeat interviews with randomly selected respondents and cross‐checking the data written in questionnaires with the information available in hospital records. Discussions were held with field workers until they reached a consensus if any differences were observed between sources of data. The control of data quality was reinforced by the continuous supervision of the field work.
Sample size
Maternal education, considered as a major determinant of perinatal mortality, provided the basis for determining sample size. The proportion of lower educational levels was estimated at 25% in the control group. With this estimate, a sample size that comprised 210 cases and 420 controls was considered sufficient for detecting an odds ratio of 1.7 with 95% confidence level and 80% power. Based on the assumption that non‐participation levels would be 10%, the sample was increased to 234 cases and 468 controls. The sample size was calculated using Epi‐Info version 3.5.1, 2008.
Analytical framework
In 2009, UNICEF developed a conceptual framework for assessing and analysing causes and determinants of maternal and neonatal mortality and morbidity 16. This framework comprises proximate, intermediate and distal levels of determinants, which are interrelated and affect mortality through complex pathways. UNICEF suggested that basic factors at societal level (distal level) affect morbidity and mortality through underlying factors at family, community and district levels (intermediate level) and more proximate factors at individual level (proximate level). This study adapted the same hierarchical scheme, which included major factors that may locally influence health outcomes.
Outcome and covariates
The study outcome was perinatal mortality, which encompassed stillbirths and early neonatal deaths. Stillbirths consisted of macerated and fresh stillbirths. A macerated stillbirth was defined as an intrauterine foetal death – the absence of a foetal heartbeat – which occurred before the onset of labour and presented with degenerative changes. A fresh stillbirth was defined as an intrauterine foetal death that occurred during labour or delivery without degenerative changes or skin maceration 17. Perinatal mortality was coded as a dichotomous variable according to the survival status of the baby.
Independent variables included maternal education and residence, household wealth and possessing health insurance. These were considered to be key measures of social differentials in perinatal mortality at the intermediate level. Maternal education was defined as the highest level of educational attainment and categorised into no formal education, primary education and secondary or higher education. Primary school consisted of 6 years, secondary school of 6 years and higher education of 2 years or more at a university or other higher learning institution. Some mothers had attended school during two different periods, and some had attended before reforms in the educational system, when primary school was based on 7 or 8 years. Maternal residence was classified as living in a rural or urban area. Household wealth was assessed using an asset score constructed through principal component analysis 18, based on the households’ possessions and facilities as reported by the mothers. These included whether that had a radio, television, electricity in the house, computer, refrigerator, mobile phone, landline phone, bicycle, motorcycle and car. The main source of their water supply was also assessed, by whether it was piped into their house, piped into the yard, a public tap, protected well, protected spring, unprotected well, unprotected spring and surface water. The main sources of cooking energy, such as electricity, natural gas, charcoal and firewood, were also included. The asset index was based on scores that were then split into quintiles. The two highest quintiles were considered as richer, the middle quintile as middle and the two lowest quintiles as poorer. Health insurance status was coded as not insured, community health insurance or state and other insurances, which included military medical and private insurances.
Other covariates such as maternal age in years (<20, 20 to 34, >34), parity (zero, one to four and more than four) and sex of the child were considered as potential determinants of perinatal mortality at the proximate level.
Data analysis
Data were summarised in a descriptive table generated from cross‐tabulations between covariates and the main outcome, which was perinatal mortality. Pearson chi‐square and Fisher's exact tests were applied to compare groups among variables of interest. Kendall's tau‐b correlation was used to check for colinearity between these variables. The mean age of the case and control mothers was compared using independent sample t‐test, and median parity was compared using the Mann–Whitney U‐test.
Conditional logistic regression was performed to analyse social differentials influencing perinatal mortality and the strength of the associations were expressed as odds ratios with 95% confidence intervals (CI). This type of logistic regression is specifically recommended for case–control designs that involve matching individuals 19. A crude analysis was used to examine the relationship between each covariate and outcome. All variables displaying a p‐value of <0.20 were retained for adjusted analysis, which also involved other variables known to be predictors of perinatal mortality 7.
The hierarchical framework described above was considered when selecting relevant variables for multiple analyses. Interactions between socio‐economic variables on the outcome were also explored. All analyses were conducted using IBM SPSS Statistics 20. The level of significance was set at a p‐value of <0.05. Data with missing values were excluded from the analyses.
The Rwanda National Ethics Committee approved this study (ethics approval number 086 RNEC/2012, 11 April 2012, Kigali, Rwanda).
Results
During the study period, there were 8424 births, with 80% taking place in the district hospital and the remaining 20% taking place in the tertiary referral hospital. Of these, 269 ended in perinatal deaths (Fig. 1). This corresponds to a perinatal mortality rate of 32 of every 1000 births (95% CI 28–36). Stillbirths and early neonatal deaths contributed to the mortality rate, accounting for 20 of every 1000 births (95% CI 17–23) and 12 of every 1000 live births (95% CI 10–15), respectively.
Figure 1.
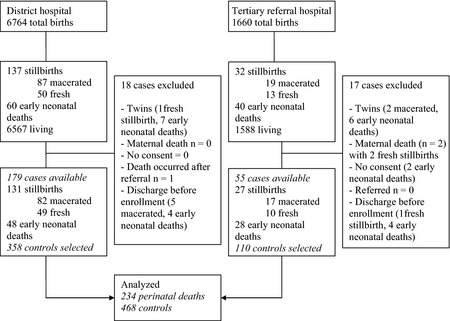
Flowchart of study population.
We excluded 35 perinatal deaths (13%) from the analyses for various reasons, including 16 twin deaths, two maternal deaths, two mothers who withheld their consent, one infant who was referred elsewhere and 14 infants who were discharged before enrolment in the study. In total, 234 singleton perinatal deaths and 468 controls were analysed (Fig. 1). The proportion of partially missing data was <0.1% and was related to maternal education.
Table 1 illustrates the socio‐economic and demographic characteristics of the mothers in the case and control groups. In the control group, 97% of the mothers lived in urban areas compared to 91% of cases and they had similar educational levels. Cases more frequently came from households of lower wealth compared to the controls (p = 0.02). Overall, 96% of mothers had used health insurance during pregnancy. The number of those using community health insurance was similar among the two groups, whereas other types of insurance were used more by mothers in the control group. The mean age of the case group mothers was 28.4 years (range 16–47), and it was 28.1 (range 15–48) in the control group (p = 0.50). The most common age group for both cases and controls was 20 to 34 years old and those with parity between one and four. The median parity of mothers of cases (median = 1, range 0–9) and controls (median = 1, range 0–6) was the same (p = 0.83). The proportion of boys was slightly higher, with an overall male to female ratio of 1.2:1 among cases and 1.1:1 among controls.
Table 1.
Socio‐economic and demographic characteristics of perinatal deaths and controls at hospitals, Kigali, Rwanda, 18 July 2012 to 8 May 2013
| Characteristics | Cases (n = 234) % (n/n) | Controls (n = 468) % (n/n) |
|---|---|---|
| Socioeconomic characteristics | ||
| Maternal residence | ||
| Urban | 91 (213/234) | 97 (455/468) |
| Rural | 9.0 (21/234) | 3.0 (13/468) |
| Maternal education | ||
| Secondary or higher | 36 (83/234) | 39 (184/464) |
| Primary | 57 (134/234) | 56 (259/464) |
| No formal education | 7.0 (17/234) | 5.0 (21/464) |
| Household wealtha | ||
| Richer | 30 (70/234) | 39 (184/468) |
| Middle | 31 (73/234) | 31 (145/468) |
| Poorer | 39 (91/234) | 30 (139/468) |
| Type of insurance | ||
| Community | 82 (193/234) | 80 (373/468) |
| State and other insurancesb | 11 (25/234) | 17 (81/468) |
| Not insured | 7.0 (16/234) | 3.0 (14/468) |
| Demographic characteristics | ||
| Maternal age at childbirth (years) | ||
| <20 | 6.0 (14/234) | 6.0 (26/468) |
| 20–34 | 71 (167/234) | 80 (377/468) |
| >34 | 23 (53/234) | 14 (65/468) |
| Mean (SD) | 28.4 (6.9) | 28.1 (5.8) |
| Parity | ||
| 0 | 42 (98/234) | 39 (184/468) |
| 1–4 | 51 (119/234) | 55 (257/468) |
| >4 | 7.0 (17/234) | 6.0 (27/468) |
| Median (range) | 2 (0 to 10) | 2 (0 to 11) |
| Sex of child | ||
| Male | 55 (128/234) | 52 (242/468) |
| Female | 45 (106/234) | 48 (226/468) |
Data are percent (n/n).
Household wealth was assessed using an asset index developed through principal component analysis 18, which provided individual scores for households’ possessions and facilities reported by mothers. The scores were divided into quintiles. Two highest quintiles were considered as ‘richer’, the middle quintile as ‘middle’ and the two lowest quintiles as ‘poorer’.
Other insurances: military medical and private insurances.
The relationship between health insurance status and other socio‐economic characteristics of controls, representing the population from which cases were generated, is reported in Table 2. State and other insurance schemes were commonly used by women with secondary or higher educational levels or living in rich households. Women with no formal education or living in households with poor or middle socio‐economic status were more likely to have no insurance or to use community health insurance. We found no or low associations between socio‐economic and proximate characteristics when checking for collinearity in the sample of controls (data not shown).
Table 2.
Relationship between health insurance status and other socio‐economic characteristics of controls at hospitals, Kigali, Rwanda, 18 July 2012 to 8 May 2013
| Socioeconomic characteristics | Health insurance status | p‐value | ||
|---|---|---|---|---|
| Not insured | Community | State and other insurancesa | ||
| Maternal residence | ||||
| Urban | 93 (13/14) | 98 (365/373) | 95 (77/81) | 0.23 |
| Rural | 7.0 (1/14) | 2.0 (8/373) | 5.0 (4/81) | |
| Maternal education | ||||
| Secondary or higher | 62 (8/13) | 27 (99/370) | 95 (77/81) | 0.01 |
| Primary | 23 (3/13) | 68 (252/370) | 5.0 (4/81) | |
| No formal education | 15 (2/13) | 5.0 (19/370) | 0.0 (0/81) | |
| Household wealthb | ||||
| Richer | 64 (9/14) | 31 (118/373) | 70 (57/81) | 0.01 |
| Middle | 7.0 (1/14) | 35 (129/373) | 19 (15/81) | |
| Poorer | 29 (4/14) | 34 (126/373) | 11 (9/81) | |
Data are percent (n/n).
Other insurances: military medical and private insurances.
Household wealth was assessed using an asset index developed through principal component analysis 18, which provided individual scores for households’ possessions and facilities reported by mothers. The scores were divided into quintiles. Two highest quintiles were considered as ‘richer’, the middle quintile as ‘middle’ and the two lowest quintiles as ‘poorer’.
The associations between socio‐economic characteristics and perinatal mortality identified from crude and adjusted analyses are shown in Table 3. In crude analysis, living in rural areas or poor households or having no insurance constituted higher risks of perinatal death. Having state and other health insurance policies was associated with even lower perinatal mortality risks than community insurance. Maternal education levels were not associated with perinatal mortality.
Table 3.
Crude and adjusted odds ratios (OR) for factors associated with perinatal mortality at hospitals, Kigali, Rwanda, 18 July 2012 to 8 May 2013
| Variables | Crudes analysesa | Adjusted analysesa | ||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Socioeconomic determinants | ||||
| Maternal residence | ||||
| Urban | Reference | |||
| Rural | 4.15 | 1.88–9.16 | 3.31 | 1.43–7.61 |
| Maternal education | ||||
| Secondary or higher | Reference | |||
| Primary | 1.17 | 0.82–1.68 | 0.73 | 0.51–2.57 |
| No formal education | 1.92 | 0.93–3.96 | 1.15 | 0.47–1.13 |
| Household wealthb | ||||
| Richer | Reference | |||
| Middle | 1.34 | 0.89–2.00 | 1.34 | 0.86–2.10 |
| Poorer | 1.74 | 1.18–2.57 | 1.53 | 0.96–2.46 |
| Type of insurance | ||||
| Community | Reference | |||
| State and other insurancesc | 0.46 | 0.25–0.84 | 0.49 | 0.25–0.95 |
| Not insured | 2.08 | 0.96–4.50 | 2.11 | 0.91–4.89 |
| Proximate determinants | ||||
| Maternal age at childbirth (years) | ||||
| <20 | 1.22 | 0.61–2.41 | 1.14 | 0.57–2.29 |
| 20–34 | Reference | |||
| >34 | 1.92 | 1.25–2.93 | 1.93 | 1.23–3.02 |
| Parity | ||||
| 0 | 1.15 | 0.82–1.61 | ||
| 1–4 | Reference | |||
| >4 | 1.35 | 0.71–2.57 | ||
| Sex of child | ||||
| Male | 1.12 | 0.82–1.53 | ||
| Female | Reference | |||
Conditional logistic regression performed for analysis of perinatal mortality risks. This type of logistic regression is adapted for a matched case–control study 19. In crude analysis, all factors were included separately in models. In adjusted analysis, socio‐economic factors (maternal education and residence, household wealth, health insurance status) were included simultaneously in model and adjusted for maternal age.
Household wealth was assessed using an asset index developed through principal component analysis 18, which provided individual scores for households’ possessions and facilities reported by mothers. The scores were divided into quintiles. Two highest quintiles were considered as ‘richer’, the middle quintile as ‘middle’ and the two lowest quintiles as ‘poorer’.
Other insurances: military medical and private insurances.
Regarding proximate determinants, only mothers aged more than 34 years old presented significant risks for perinatal death. There were no significant differences in perinatal mortality related to either parity or sex of the child.
After adjustments, living in rural areas or having no insurance remained associated with increased risks of perinatal death. State and other health insurance schemes still showed reduced risk of perinatal mortality compared to community health insurance. The association between living in poor households and perinatal mortality was no longer significant. Perinatal deaths were still significantly higher for women over 34 years old.
We also tested whether there were any significant interactions between the included socio‐economic characteristics, but no evidence of this was found (p > 0.10).
Discussion
This study examined whether there were social differentials in perinatal mortality in hospitals in Kigali, Rwanda. It showed that living in a rural area or not having insurance was associated with an increased risk of perinatal mortality. There was no association between maternal education levels and this outcome. Similarly, there was no significant association with household wealth.
This is the first study to report on social disparities in perinatal mortality in Rwanda. As this study was performed in two major hospitals, it does not provide a representative picture of the country, but it does demonstrate the situation at higher levels in the health system. The sample size was statistically determined based on the perinatal mortality data that were locally available. Controls were matched with cases by day and place of birth to minimise selection bias. Recall bias was avoided by collecting data soon after birth, before discharge from hospital.
However, some limitations should be discussed. For example, there was a borderline association observed between household wealth and perinatal survival. Also, all cases of death were not captured. Women were usually discharged 12 h after delivery, and most dead babies were taken away from the hospital within a few hours after death. In some cases, data collectors were informed after discharge, especially if they occurred at night or over a weekend. There was a potential risk of misclassification between miscarriages, early foetal deaths and stillbirths, especially when mothers were unaware of the date of their last menstrual period. To minimise this risk, a cut‐off weight of 500 g distinguished the two groups and each baby was weighed after birth. In addition, an ultrasound was performed in almost all cases of suspected stillbirths before delivery to assess foetal heartbeat, malformations and provide estimates of the age and weight of foetus and other necessary information.
Misclassification between macerated and fresh stillbirths, which may be due to the lack of information about the onset of labour or poor assessment of skin deterioration signs, was another potential bias. The risk of misclassification could also have applied to some cases of early neonatal deaths, which might have been considered as fresh stillbirths. However, it was unlikely that misclassification among stillbirths, or between stillbirths and early neonatal deaths, would have affected our results as the analyses focused on perinatal deaths, which included both categories.
We constructed an asset score based on household assets used in Rwandan demographic and health surveys 10. However, not all items were considered when calculating this score. The characteristics of roofs, walls and floors of houses and families’ toilets would have required observations at home and that was not feasible.
This hospital‐based study mainly included an urban population. Therefore, our findings cannot be generalised to the whole country because of different socio‐economic characteristics between rural and urban areas 10.
The risk of perinatal mortality was increased in rural areas, and this finding is consistent with earlier studies conducted in other countries 20, 21. One explanation might lie in limited access to adequate healthcare services for pregnant women living in rural areas 22. In addition, some women from rural areas were referred from other district hospitals some distance from Kigali for emergency obstetric care. The unfavourable foetal or neonatal outcomes experienced by these women could also be explained by the distance from the hospital or delay in referral.
Maternal education levels are frequently associated with perinatal survival 7. In contrast, we found no significant differences in perinatal mortality risks between mothers with different educational levels. Our research confirms other findings reported from elsewhere in low‐income countries 23, 24. The Rwandan government has undertaken to improve the accessibility and quality of health services as well as the education of the population in recent years. This may have helped to reduce health inequalities related to education, as reflected in the lack of association between different maternal educational levels and perinatal mortality. However, these findings should be cautiously interpreted as mothers with no formal education were under‐represented in this study (Table 1).
Our results indicated that household wealth was associated with perinatal survival in crude analyses. However, after adjusting for other covariates, the difference in mortality risk between poor and rich households was of borderline statistical significance. Previous investigations have indicated that neonatal mortality was not influenced by household wealth in a country like Vietnam, where strategies to address the rich–poor divide have been strengthened 25. Similarly, interventions aimed at reducing the gap between poor and wealthy people were scaled up in Rwanda. These interventions included cash transfers for access to education and health, direct money grants to extremely poor individuals unable to work, building shelters, income generating projects, access to investment loans and other poverty‐alleviating strategies 15. Our finding highlights the need to strengthen such initiatives to further reduce the social gaps in perinatal mortality. However, some reservations could be raised about relatively low study power, which might not have allowed us to detect differences in perinatal mortality between rich and poor families after adjustments (Table 3).
Our analysis shows that the patterns of perinatal mortality were related to the health insurance status of mothers. Women with no insurance experienced an increased risk of perinatal death. Previous studies have indicated that having health insurance improved healthcare utilisation in Rwanda 26 and other countries 27. Improved access to health care could also result in better perinatal outcomes 28.
However, having insurance may not guarantee equal access to health care and subsequent health outcomes 27, 29. Other socio‐economic characteristics, such as maternal education and household wealth, were also recognised as key determinants of healthcare utilisation 30. Our study revealed that these factors were associated with the type of insurance used during pregnancy (Table 2), which may explain the different protective effects observed between community health insurance and state and other insurance schemes on perinatal mortality. Various extra costs, including transport fees and purchasing drugs and other commodities not covered by insurance schemes may be an economic burden, especially for poor families with limited access to adequate health care.
Furthermore, mothers covered by community health insurance were not as likely to use the same health facilities during pregnancy as other mothers insured by state and other insurance schemes. Mothers with community health insurance were expected to seek health care in public institutions and follow referral steps to be covered by insurance. These restrictions were not applied to mothers using state or other insurance schemes, as they could choose health facilities and seek appropriate care.
Conclusion
A number of factors have reduced the gaps between health outcomes in rich and poor people in Rwanda in recent years. These include improving access to formal education, strengthening the health system – with special emphasis on universal coverage by health insurance – together with targeted interventions for those in need. These efforts may also have favourably influenced social differentials in perinatal mortality. Our findings show no or small inequalities in perinatal survival in relation to levels of maternal education and household wealth. However, women living in rural areas or having no insurance had increased risks of perinatal death. These results may be useful for guiding development of future perinatal health and survival initiatives that lead to safe pregnancy and childbirth and strategies to overcome barriers to equal access to adequate health care.
Funding
This study was funded by the Swedish International Development Cooperation Agency (Sida) and the Faculty of Medicine, Uppsala University.
Competing Interests
The authors declare that they have no competing interests.
Acknowledgements
The authors would like to thank Dr Théobard Hategekimana and Dr Patrick Mulindwa, the directors of two hospitals for permission to conduct the study and for their support. We also wish to acknowledge the mothers who participated in this study, the research assistants involved in data collection and the health workers in both hospitals for facilitating the fieldwork.
References
- 1. Cousens S, Blencowe H, Stanton C, Chou D, Ahmed S, Steinhardt L, et al. National, regional, and worldwide estimates of stillbirth rates in 2009 with trends since 1995: a systematic analysis. Lancet 2011; 377: 1319–30. [DOI] [PubMed] [Google Scholar]
- 2. Lozano R, Wang H, Foreman KJ, Rajaratnam JK, Naghavi M, Marcus JR, et al. Progress towards Millennium Development Goals 4 and 5 on maternal and child mortality: an updated systematic analysis. Lancet 2011; 378: 1139–65. [DOI] [PubMed] [Google Scholar]
- 3. WHO . Neonatal and perinatal mortality: country, regional and global estimates.2006. Available at: http://whqlibdoc.who.int/publications/2006/9241563206_eng.pdf (accessed February 13, 2014).
- 4. UN Inter‐agency Group for Child Mortality Estimation . Levels and trends in child mortality report 2013. 2013. Available at: http://www.who.int/maternal_child_adolescent/documents/levels_trends_child_mortality_2013.pdf?ua=1 (accessed January 22, 2014).
- 5. Essen B, Bodker B, Sjoberg NO, Langhoff‐Roos J, Greisen G, Gudmundsson S, et al. Are some perinatal deaths in immigrant groups linked to suboptimal perinatal care services? BJOG 2002; 109: 677–82. [PubMed] [Google Scholar]
- 6. Ravelli AC, Tromp M, Eskes M, Droog JC, van der Post JA, Jager KJ, et al. Ethnic differences in stillbirth and early neonatal mortality in The Netherlands. J Epidemiol Community Health 2010; 65: 696–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Andargie G, Berhane Y, Worku A, Kebede Y. Predictors of perinatal mortality in rural population of Northwest Ethiopia: a prospective longitudinal study. BMC Public Health 2013; 13: 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. WHO . Closing the gap in a generation: health equity through action on the social determinants of health. 2008. Available at: <http://whqlibdoc.who.int/publications/2008/9789241563703_eng.pdf?ua=1> (accessed April 20, 2014). [DOI] [PubMed]
- 9. National Institute of Statistics of Rwanda, Ministry of Health, and ICF International . Rwanda demographic and health survey 2005. 2006. Available at: http://www.statistics.gov.rw/publications/rwanda-demographic-and-health-survey-report-dhs-2005 (accessed February 25, 2014).
- 10. National Institute of Statistics of Rwanda, Ministry of Health, and ICF International . Rwanda demographic and health survey 2010. 2012. Available at: http://statistics.gov.rw/publications/demographic-and-health-survey-2010-final-report (accessed February 19, 2014).
- 11. Republic of Rwanda Ministry of Health . Health sector strategic plan July 2009–June 2012. 2009. Available at: https://www.google.rw/url?sa=t&rct=j&q=&esrc=s&source=web&cd=4&ved=0CDkQFjAD&url=http%3A%2F%2Fwww.ipar-rwanda.org%2Findex.php%3Foption%3Dcom_docman%26task%3Ddoc_download%26gid%3D91%26Itemid%3D151&ei=eCnxUs2uBoeBywODqoDQAw&usg=AFQjCNGwEGjO83oXwYD22k9wyaPwPg90cg (accessed February 5, 2014).
- 12. Republic of Rwanda Ministry of Health . National reproductive health policy. 2003. Available at: <http://www.africanchildforum.org/clr/policy%20per%20country/rwanda/rwanda_reproductivehealth_2003_en.pdf> (accessed April 18, 2014).
- 13. Republic of Rwanda Ministry of Health . Rwanda national health insurance policy. 2010. Available at: http://www.moh.gov.rw/fileadmin/templates/Docs/insurance_policy1.pdf (accessed April 17, 2014).
- 14. Abbott P, Rwirahira J. Millennium development goals: progress report on Rwanda. 2010. Available at: http://www.abdn.ac.uk/sustainable-international-development/documents/UNDP%20final%20PA%2030%20October%202010_1.pdf (accessed February 2, 2014).
- 15. Republic of Rwanda Ministry of Local Government . National social protection strategy. 2011. Available at: http://www.ilo.org/gimi/gess/ShowRessource.action?ressource.ressourceId=23208 (accessed February 12, 2014).
- 16. UNICEF . The state of the world's children 2009: maternal and newborn health. 2009. Available at: http://www.unicef.org/sowc09/docs/SOWC09-FullReport-EN.pdf (accessed April 20, 2014).
- 17. WHO . Safe motherhood needs assessment. 2001. Available at: <http://whqlibdoc.who.int/hq/2001/WHO_RHT_MSM_96.18_Rev.1_Pt.1.pdf?ua=1> (accessed February 24, 2014).
- 18. Gwatkin DR, Rustein S, Johnson K, Pande RP, Wagstaff A. Socioeconomic differences in health nutrition and population in Bangladesh. 2000. Available at: http://siteresources.worldbank.org/INTPAH/Resources/Publications/Country-Reports/bangladesh.pdf (accessed February 26, 2014).
- 19. Petrie A, Sabin C. Medical statistics at a glance, 3rd ed New York: Wiley‐Blackwell, 2009: 90–1. [Google Scholar]
- 20. Tromp M, Eskes M, Reitsma JB, Erwich JJ, Brouwers HA, Rijninks‐van Driel GC, et al. Regional perinatal mortality differences in the Netherlands; care is the question. BMC Public Health 2009; 9: 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nankabirwa V, Tumwine JK, Tylleskar T, Nankunda J, Sommerfelt H. Perinatal mortality in eastern Uganda: a community based prospective cohort study. PLoS ONE 2011; 6: e19674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Houweling TA, Ronsmans C, Campbell OM, Kunst AE. Huge poor‐rich inequalities in maternity care: an international comparative study of maternity and child care in developing countries. Bull World Health Organ 2007; 85: 745–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fawole AO, Shah A, Tongo O, Dara K, El‐Ladan AM, Umezulike AC, et al. Determinants of perinatal mortality in Nigeria. Int J Gynaecol Obstet 2011; 114: 37–42. [DOI] [PubMed] [Google Scholar]
- 24. Schmiegelow C, Minja D, Oesterholt M, Pehrson C, Suhrs HE, Bostrom S, et al. Factors associated with and causes of perinatal mortality in northeastern Tanzania. Acta Obstet Gynecol Scand 2012; 91: 1061–8. [DOI] [PubMed] [Google Scholar]
- 25. Hoa DP, Nga NT, Malqvist M, Persson LA. Persistent neonatal mortality despite improved under‐five survival: a retrospective cohort study in northern Vietnam. Acta Paediatr 2008; 97: 166–70. [DOI] [PubMed] [Google Scholar]
- 26. Saksena P, Antunes AF, Xu K, Musango L, Carrin G. Mutual health insurance in Rwanda: evidence on access to care and financial risk protection. Health Policy 2010; 99: 203–9. [DOI] [PubMed] [Google Scholar]
- 27. Devoe JE, Baez A, Angier H, Krois L, Edlund C, Carney PA. Insurance + access not equal to health care: typology of barriers to health care access for low‐income families. Ann Fam Med 2007; 5: 511–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee AC, Lawn JE, Cousens S, Kumar V, Osrin D, Bhutta ZA, et al. Linking families and facilities for care at birth: what works to avert intrapartum‐related deaths? Int J Gynaecol Obstet 2009; 107(Suppl. 1): S65–85, S86–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Skinner AC, Mayer ML. Effects of insurance status on children's access to specialty care: a systematic review of the literature. BMC Health Serv Res 2007; 7: 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hong R, Ayad M, Ngabo F. Being insured improves safe delivery practices in Rwanda. J Community Health 2011; 36: 779–84. [DOI] [PubMed] [Google Scholar]


