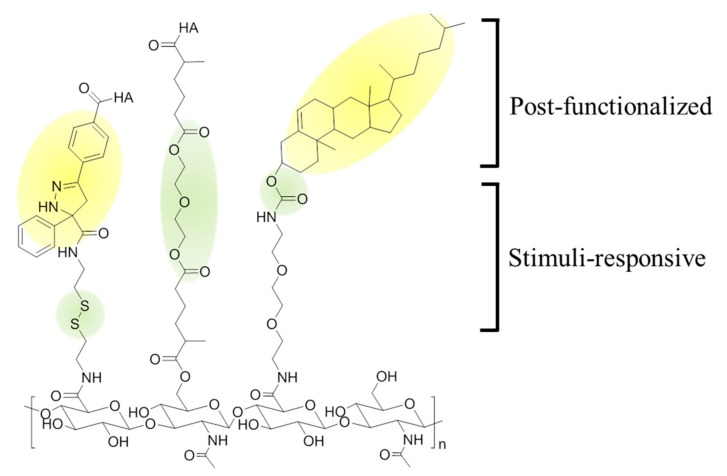
Figure 6.
Chemical modification of hyaluronic acid (HA) with (1) functionality groups and (2) stimuli-responsive moieties. Photo-crosslinking between tetrazole and methacrylate generates a durable nanogel structure, while disulfide bonds endows degradability to nanogels under reductive condition (Left). A crosslinker functions as not only the precursor of the nanogel, but also enzyme-responsive moiety, such as ester group (Middle). To make the nanogels using hydrophilic HA, a hydrophobic moiety can be post-functionalized on the backbone. (i.e., cholesterol) (Right).