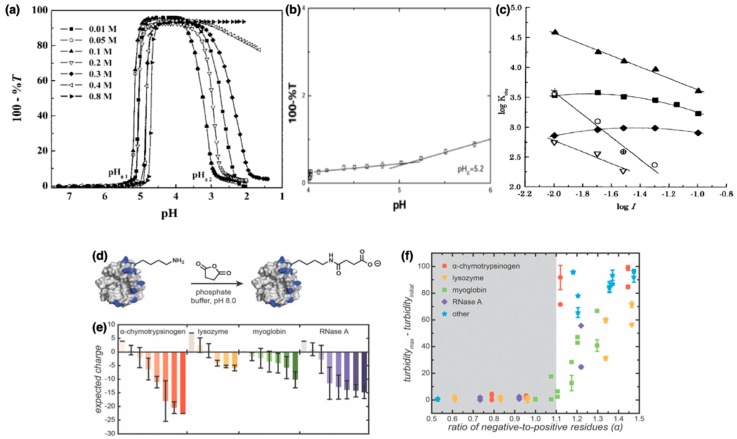
Figure 3.
(a) turbidity of complexes of BLG with pectin with varying pH at different NaCl concentrations, where pHϕ1 and pHϕ2 denote pH at aggregation and dissolution of complexes. Reprinted with permission from Ref. [91]. Copyright 2007, American Chemical Society. (b) turbidity of BSA-PDADMAC as a function of pH at 50 mM salt, where pHC denotes pH at which soluble complexes begin to form. Reprinted with permission from Ref. [92] Copyright 2010, American Chemical Society; (c) binding constants of BLG-NaPSS and BLG-PAMPS as a function of salt concentration: (Filled diamond) BLG-NaPSS at pH 7.0; (Filled square) BLG-NaPSS at pH 6.7; (Filled triangle) BLG-NaPSS at pH 6.3; (Open inverted triangle) BLG-PAMPS at pH 6.3; (Open Circle) BLG-PAMPS at pH 6.1; (Addition symbol) BLG-PAMPS at pH 6.1. Reprinted with permission from Ref. [76] Copyright 2003, American Chemical Society; (d) schematic for supercharging of proteins by succinic anhydride; (e) the expected charge on modified proteins with varying degrees of supercharging; (f) change in turbidity as a function of ratio of negative to positive charge residues on proteins. Grey area depicts complexes which do not phase separate; (d), (e) and (f) are reproduced from Ref. [93] with permission from the Royal Society of Chemistry.