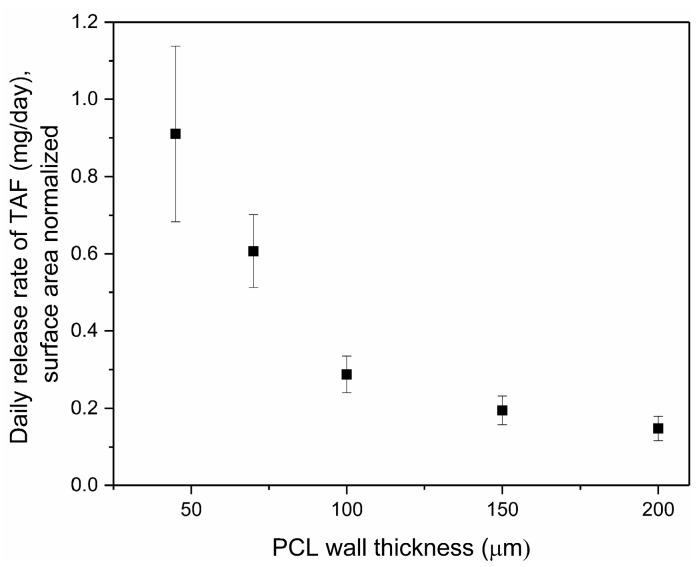
Figure 3.
Daily release of TAF (mg/day) from implants with different wall thicknesses as calculated over 35 consecutive days within an in vitro assay. Implants comprised a 2:1 TAF-castor oil formulation (dimensions: 2.5 mm outer diameter (OD) by 40 mm length) fabricated with Sigma-Grade PCL. Three implants were tested per condition.