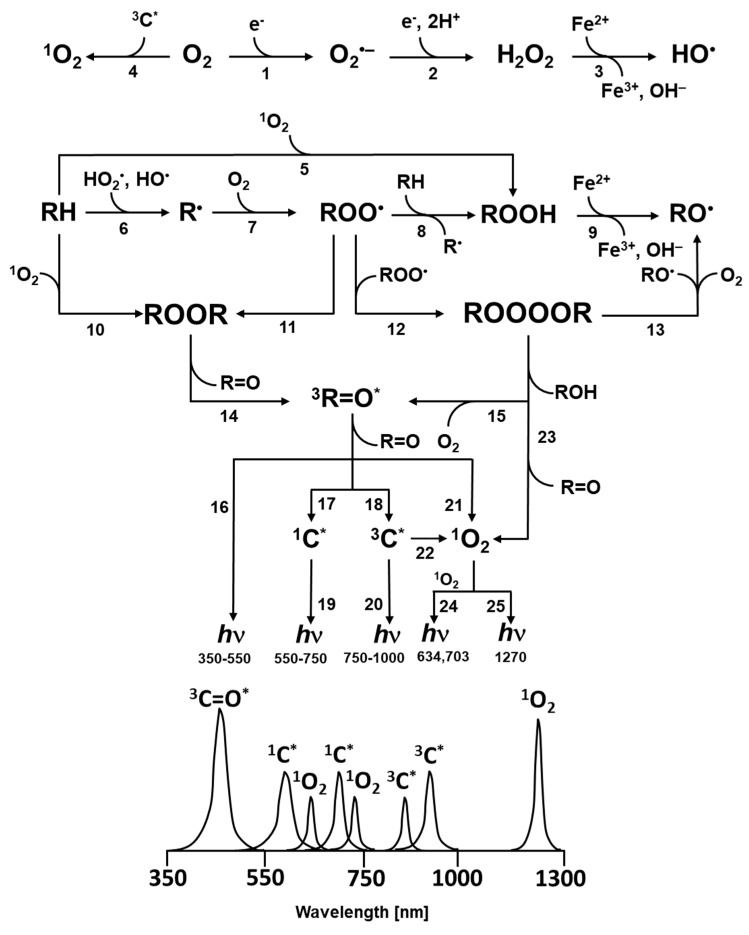
Figure 1.
Mechanism of the formation of electronically excited species by oxidative metabolic processes. One-electron reduction of molecular oxygen by highly reducing species forms O2•- (reaction 1). The dismutation of O2•- generates H2O2 (reaction 2), whereas the subsequent one-electron reduction of H2O2 leads to the formation of HO• (reaction 3). The triplet-triplet energy transfer from triplet chromophore to molecular oxygen results in the formation of 1O2 (reaction 4). Formation of ROOH from R and 1O2 via ene reaction (reaction 5). The hydrogen abstraction from biomolecules (lipids, proteins and nucleic acids) (RH) by radical ROS (HO•, HO2•) generates R• (reaction 6). The subsequent one-electron oxidation of R• brings about the formation ROO• (reaction 7). The consequent hydrogen abstraction from another R by ROO• forms ROOH (reaction 8). The one-electron reduction of ROOH results in the formation of RO• and OH− (reaction 9). ROOR is formed by either the cycloaddition of 1O2 (reaction 10) or the cyclization of ROO• (reaction 11). ROOOOR is formed by the recombination of two ROO• (reaction 12). The decomposition of ROOR (reaction 14) or ROOOOR (reaction 15) results in the formation of 3R=O*. Alternatively, the decomposition of ROOOOR can lead to the formation of two RO• and O2 (reaction 13). The electronic transition from 3R=O* to R=O is accompanied by the photon emission (16). The energy transfer from 3R=O* to chromophores results in the formation of 1C* (reaction 17) and 3C* (reaction 18) chromophores. The electronic transition from 1C* and 3C* to the ground state of chromophores is accompanied by the photon emission. (reaction 19, 20). The triplet-triplet energy transfer from 3R=O* (reaction 21) and 3C* (reaction 22) to molecular oxygen forms (1O2). Alternatively, the decomposition of ROOOOR via the Russell-type mechanism, results in 1O2 (reaction 23) with dimol photon emission (reaction 24) and monomol photon emission (reaction 25).