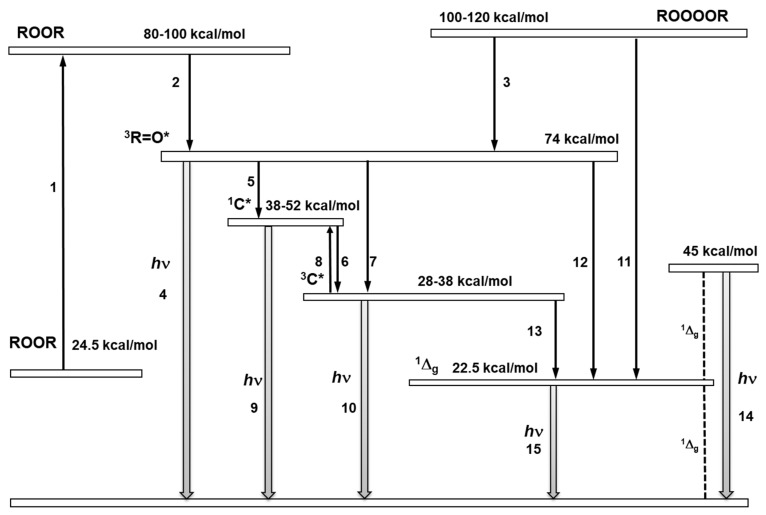
Figure 7.
Schematic energy level diagram of the formation of electronically excited species through the decomposition of ROOR and ROOOOR. The decomposition of ROOR occurs via a transition state (reaction 1). The decomposition of the transition state of ROOR generates 3R=O* (reaction 2). The decomposition of ROOOOR to 3R=O* (reaction 3). The electronic transition from the triplet energy level of 3R=O* to the ground state is accompanied by light emission (reaction 4). The triplet-singlet energy transfer from 3R=O* to 1C* (reaction 5). The triplet-triplet energy transfer from 3R=O* to chromophore 3C* (reaction 6). Intersystem crossing from 1C* to 3C* (reaction 6). The reverse intersystem crossing converts 3C* to 1C* (reaction 8). The electronic transition from 1C* and 3C* to the ground state is accompanied by photon emission (reaction 9,10). The decomposition of ROOOOR generates singlet oxygen (1Δg) (reaction 11). The triplet-singlet energy transfer from 3R=O* to molecular oxygen is feasible (reaction 12). The electronic transition from the energy level of singlet oxygen (1Δg) to the triplet energy level of ground state is accompanied by dimol (reaction 14) and monomol photon emission (reaction 15).