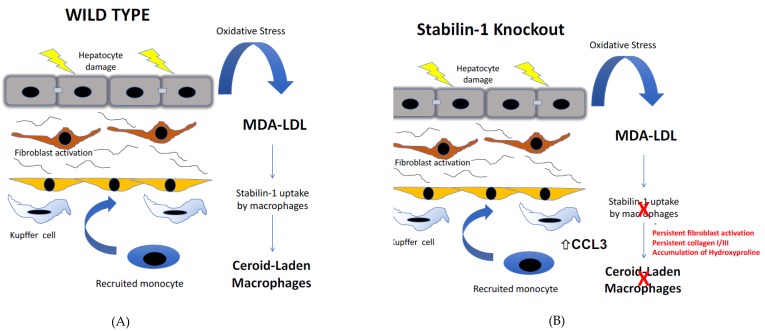
Figure 5.
Stabilin-1 expression on hepatic macrophages protects against excessive tissue damage from chronic oxidative stress. (A) In models of chronic liver injury, the repetitive damage to hepatocytes leads to oxidative stress and lipid peroxidation, which leads to the formation of malondialdheyde-lipoproteins (MDA-LDL), which accumulate in the liver. Stabilin-1 expression on hepatic macrophages leads to the uptake of MDA-LDL, which leads to the formation of ceroid-laden macrophages that are found at sites of scarring. The active uptake of MDA-LDL by stabilin-1 positive macrophages suppresses the release of pro inflammatory mediators such as CCL3. (B) In the setting of stabilin-1 deficiency, there is a loss of these ceroid-laden macrophages and a lack of accumulation of MDA-LDL within hepatic macrophages. The stabilin-1-deficient hepatic macrophages are shifted to a pro-inflammatory phenotype including excessive release of CCL3, and this is associated with excessive scarring from activated liver fibroblasts and delayed healing after liver injury.