Figure 1.
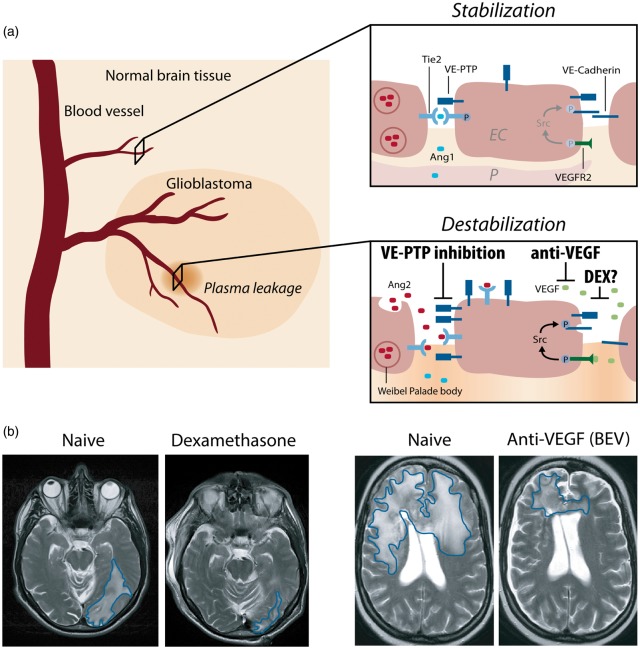
Interference of angiogenic growth factor signaling with junctional proteins and vascular leakage in GBM. (a) In the normal brain vasculature, paracrine Ang-1 signaling leads to the phosphorylation and subsequent translocation of Tie2 to endothelial cell junctions (EC junctions; upper right panel). VEGF receptor 2 (VEGFR2) is activated due to low amounts of the activating ligand VEGF, but a stable vasculature is maintained by concomitant Tie2 activation. In GBM, several mechanisms lead to the induction of vascular leakage: (1) Ang-2 is secreted from Weibel–Palade bodies and acts in an autocrine manner on Tie2, thereby blocking the Ang1-induced Tie2 phosphorylation and vessel stabilization (lower right panel). (2) Tie2 phosphorylation is further reduced by the activity of VE-PTP that dephosphorylates Tie2 at EC junctions, thereby promoting vascular permeability. VE-PTP expression is increased in hypoxic endothelial cells and in neovascular endothelial cells in mice with ischemic retinopathy suggesting increased expression in tumor vessels.76 (3) Local VEGF levels are increased leading to vascular endothelial cadherin (VE-cadherin) phosphorylation and internalization in an SRC kinase-dependent manner. Dexamethasone interferes with and prevents vascular leakage by a yet unknown mechanism that may include inhibition/downregulation of VEGF, whereas anti-VEGF treatment (BEV) neutralizes excess VEGF, and VE-PTP inhibition (AKB-9778) reduces the catalytic activity of VE-PTP to restore Tie2 activation to prevent vascular leakage. Illustration: Visual Science Communication. (b) Illustration of brain edema in GBM patients. Upon DEX treatment (left), the T2 weighted cMRI signal shows the anti-edema effect of DEX when compared with the DEX naive scenario in a GBM patient with perifocal edema (encircled in blue) in the left parietooccipital lobe. Upon BEV (anti-VEGF) therapy of another GBM patient (right), a weighted cMRI axial section shows decreased T2 signal enhancement demonstrating the anti-edema effect of BEV compared to progressive disease and perifocal edema (encircled in blue) of the corpus calosum before treatment.