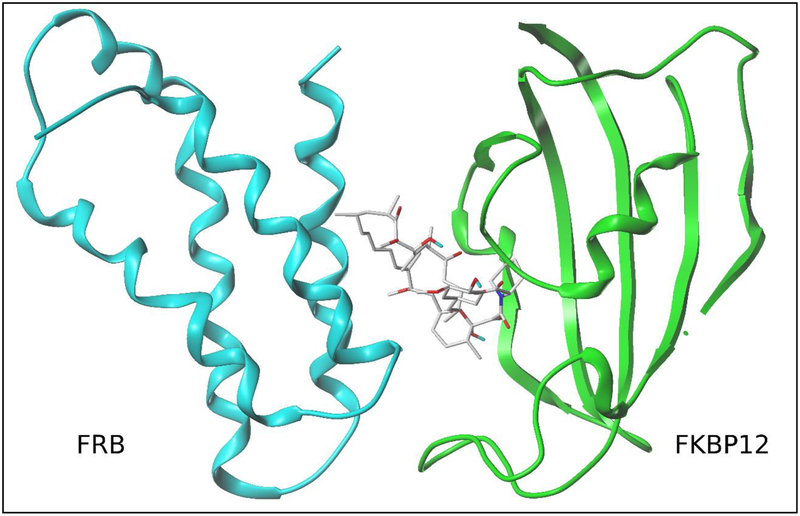
Figure 2: The mechanism of action of rapamycin and rapalogs.
Rapamycin (also known as sirolimus) is a potent immunosuppressive and anti-cancer agent that binds two proteins: FK506-binding protein (FKBP12) and FKBP-rapamycin-associated protein (FRAP). The 2.7°A structure (pdb:1FAP)18of the complex between the FRB domain of mTOR (cyan), rapamycin and FKBP12 (green) is shown. Rapamycin mediates FKBP12 dimerization with mTOR, which then blocks access to the mTOR kinase active site located in a deep cleft and hydrophobic aromatic pocket behind the FKBP12-rapamycin binding domain. Rapamycin binding to FKBP12 and subsequent selective association of the FKBP12-rapamycin complex with mTORC1 conveys highly sensitive and targeted mTORC1 inhibition. Chemical structures of sirolimus and the rapalogs (e.g. everolimus, temsirolimus, and ridaforolimus) all share a ‘central macrolide’ structure and have unique R groups at the C40 position implicating a common mechanism of mTOR inhibition [18]
Choi J, Chen J, Schreiber SL, Clardy J. Structure of the FKBP12-rapamycin complex interacting with the binding domain of human FRAP. Science. 1996;273(5272):239–242.