Drug Summary
| Drug name | Phase | Indication | Mechanism of action (rsp) | Route of administration (rsp) | Chemical structure | Pivotal trial |
|---|---|---|---|---|---|---|
| Everolimus | Dose escalation Phase I | Patients with refractory advanced solid tumors including PDAC. | mTORC1 inhibitor | Oral |
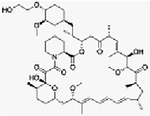 (C53H83NO14) |
31 |
| Everolimus | Phase II | GR metastatic PDAC | mTORC1 inhibitor | Oral | C53H83NO14 | 32 |
| Everolimus | Phase II | PIK3CA amplified/mutated and/or PTEN loss in advanced refractory solid tumors. | mTORC1 inhibitor | Oral | C53H83NO14 | 33 |
| Everolimus + erlotinib | Phase II | Advanced PDAC | mTORC1 inhibitor + EGFR inhibitor | Oral + oral | C53H83NO14 +  (C22H23N3O4) |
34 |
| Capecitabine + cetuximab + everolimus | Phase I/II | Advanced PDAC | Thymidylate synthase inhibitor + EGFR inhibitor + mTORC1 inhibitor | Oral + IV + oral |
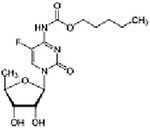 (C15H22FN3O6) + Chimeric antibody C53H83NO14 |
35 |
| Capecitabine + everolimus | Followup phase II | Advanced PDAC | Thymidylate synthase inhibitor + mTORC1 inhibitor | Oral + oral | C15H22FN3O6 C53H83NO14 |
37 |
| Everolimus + gemcitabine + cisplatin | Phase I, (3+3) | Advanced solid tumors | mTORC1 inhibitor + cytotoxic + cytotoxic | Oral + IV + IV | C53H83NO14+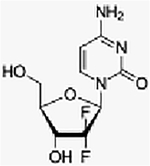 (C9H11F2N3O4) + [Pt(NH3)2Cl2] |
38 |
| Everolimus+ gemcitabine |
Phase I | Advanced PDAC | mTORC1 inhibitor + cytotoxic | Oral + IV | C53H83NO14 C9H11F2N3O4 |
39 |
| Everolimus + trametinib | Phase Ib | Advanced refractory solid tumors | mTORC1 inhibitor + MEK inhibitor | Oral + oral | C53H83NO14+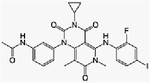 (C26H23FIN5O4) |
41 |
| Everolimus + ribociclib | Phase I (3+3) | Advanced PDAC refractory to 5-fluorouracil (5-FU) and gemcitabine-based chemotherapy | mTORC1 inhibitor + CDK4/6 inhibitor | Oral + oral | C53H83NO14 +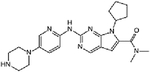 (C23H30N8O) |
42 |
