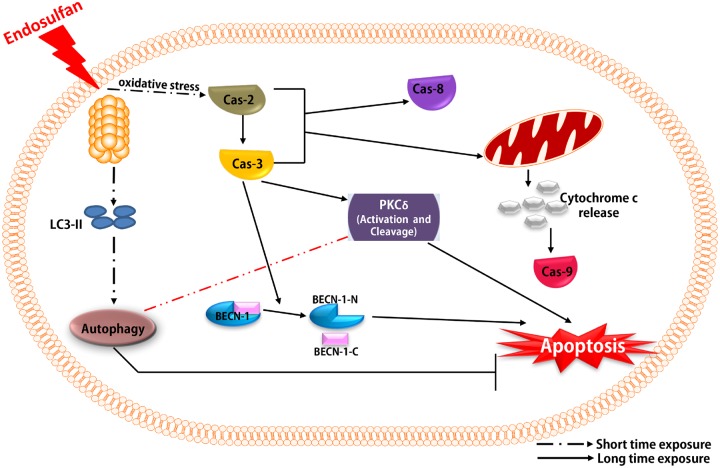
Figure 10.
Schematic representation of mechanisms underlying endosulfan-induced autophagy and apoptosis. Exposure to the environmental neurotoxicant endosulfan instantly induces autophagic processes as an early response, followed by apoptotic cell death after prolonged treatment via activation of caspases and the mitochondrion-dependent pathway. Caspase-2, as an initial caspase, is a key upstream factor mediating endosulfan-induced cell apoptosis. Caspase-2 activates the executioner caspase-3. We clearly demonstrate that autophagy is a protective process that retards endosulfan-mediated cell death. Interestingly, we also provide evidence to show that Beclin-1 can be cleaved by caspases during endosulfan-induced cell apoptosis, suggesting a potentially important role of Beclin-1 in dopaminergic neuronal cell death. The autophagic response assumes a protective role against endosulfan-mediated neurotoxicity and dopaminergic neuronal cell death. We also show that endosulfan-induced apoptosis is caspase-dependent with caspase-2, as the initiator caspase, acting upstream of the mitochondria pathway, being of central importance. We also suggest a potentially essential role of Beclin-1 cleavage in endosulfan-induced neuronal cell loss.