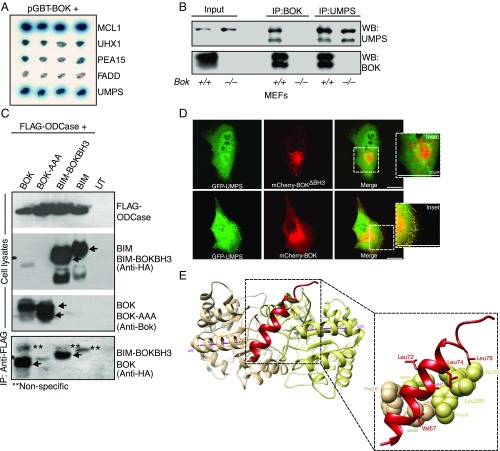
Fig. 1.
BOK interacts with UMPS through its BH3 domain. (A) Yeast 2 hybrid assay showing that BOK specifically interacts with UMPS. MCL-1 was used as a positive control, whereas Uhx1, Pea 15, and FADD are used as negative controls. (B) Immunoprecipitation analysis of BOK-UMPS interaction in MEFs at physiological levels of expression. (C) The BH3 domain of BOK mediates the interaction. Mutating the BH3 domain (BOK-AAA = L72A,R73A,L74A) abolishes the interaction (lane 2), and a chimeric BIM mutant with the BOK-BH3 domain could interact with the ODCase domain, whereas BIM by itself does not. (D) Confocal microscopy of MDCK cells expressing GFP-tagged UMPS either with mCherry-tagged BOK-ΔBH3 (Top) or BOK WT (Bottom). (E) Molecular modeling of the BOK-BH3 domain docking on ODCase. Cartoon diagram of BOK BH3 docked to ODCase dimer, viewed down the 2-fold symmetry axis (Top). Protein docking predictions place BOK (red) at the dimer interface between α2b helices (Bottom) from both monomers (monomers are colored tan and khaki, respectively, with α2b helix axis in magenta).