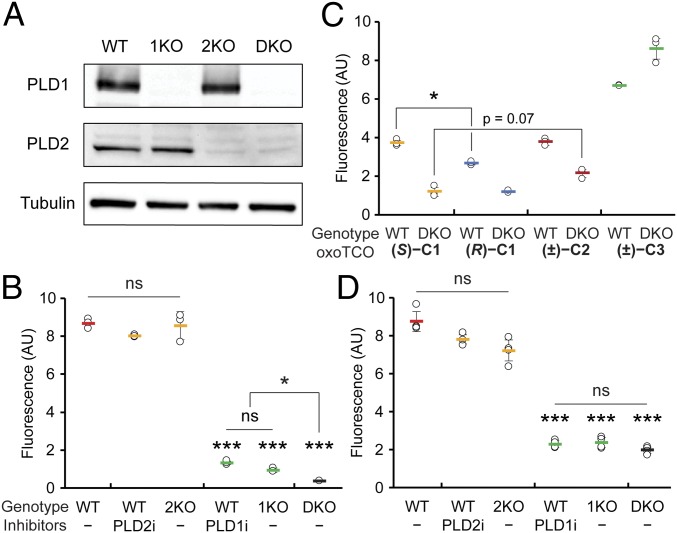
Fig. 3.
oxoTCO alcohols are effective and selective reporters of endogenous human PLD activity. (A and B) Generation and validation of PLD1 knockout (1KO), PLD2 knockout (2KO), or PLD1/2 double knockout (DKO) HeLa cells made by using CRISPR/Cas9-mediated mutagenesis. (A) Knockout was verified by Western blot analysis for PLD1, PLD2, or as a loading control, α-tubulin. (B) The indicated cells were pretreated with the indicated PLD inhibitor (PLD1i, VU0359595; or PLD2i, VU0364739) or DMSO for 30 min, followed by transphosphatidylation with 3-azido-1-propanol (1 mM) under PMA stimulation (100 nM) for 20 min, rinsing with PBS solution, and labeling with a cyclooctyne–BODIPY conjugate (1 µM) for 10 min, rinsing for 20 min, and analysis by flow cytometry. Indicated are mean fluorescence intensities in arbitrary units (AU). (C and D) Evaluation of oxoTCO alcohols as selective probes for endogenous PLD enzymes. Shown are mean fluorescence intensities from flow cytometry experiments wherein IMPACT labeling was performed as in A, except using the indicated oxoTCO alcohol (C) or (S)-oxoTCO–C1 (3 mM, 5 min; D) in place of 3-azido-1-propanol and Tz–BODIPY (0.33 µM, 1 min, no subsequent rinse) in place of cyclooctyne–BODIPY. Statistical significance was assessed by using a 1-way ANOVA followed by Games–Howell post hoc analysis. Asterisks directly above data points denote statistical significance compared with WT without the presence of inhibitors, asterisks above horizontal lines denote statistical significance comparing the 2 indicated data groups, and error bars represent SD (*P < 0.05 and ***P < 0.001; ns, not significant).