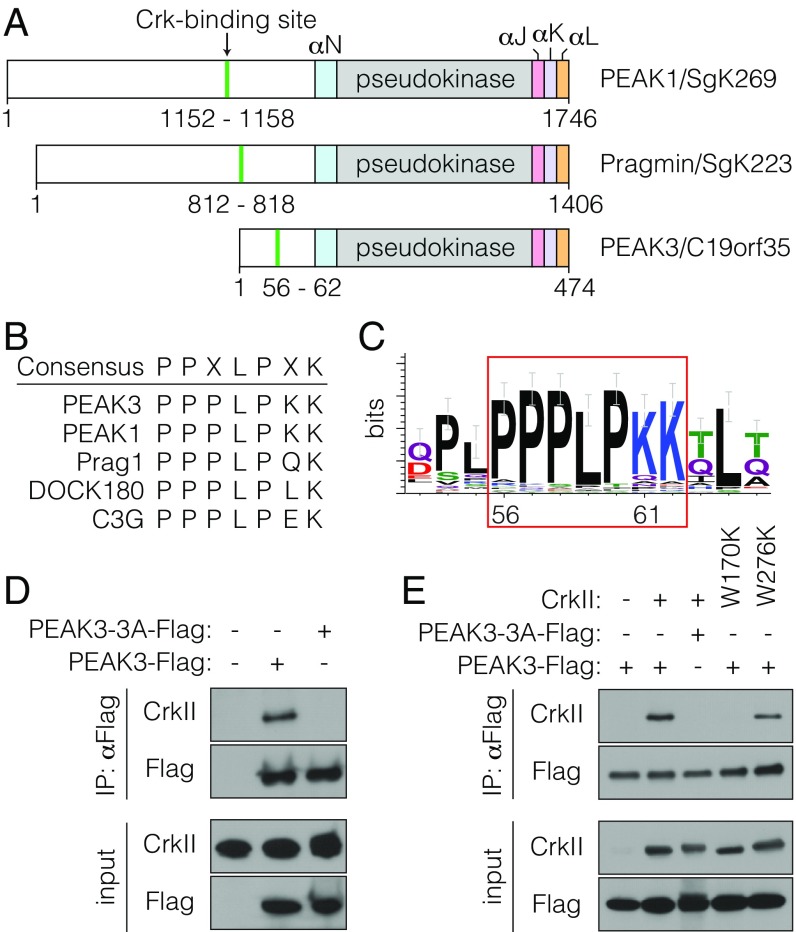
Fig. 2.
PEAK3 binds CrkII. (A) Schematic representation of PEAK1, Pragmin, and PEAK3 domain structure. The locations of the CrkII-binding sites and helical regions within the SHED domain are highlighted. (B) Consensus sequence of CrkII-binding sites in selected proteins. (C) Sequence logo depicting conservation of the CrkII-binding site in PEAK3 homologs. (D and E) Coimmunoprecipitation of FLAG-tagged wild-type PEAK3 or a CrkII-binding mutant (PEAK3-3A) transiently expressed in HEK293 cells with either endogenous CrkII (D) or transiently expressed untagged CrkII variants carrying mutations in the SH3 domains (W170K in SH3N and W276K in SH3C) (E). Protein levels were detected with the indicated antibodies by Western blot. All coimmunoprecipitation data are representative of at least 3 independent experiments.