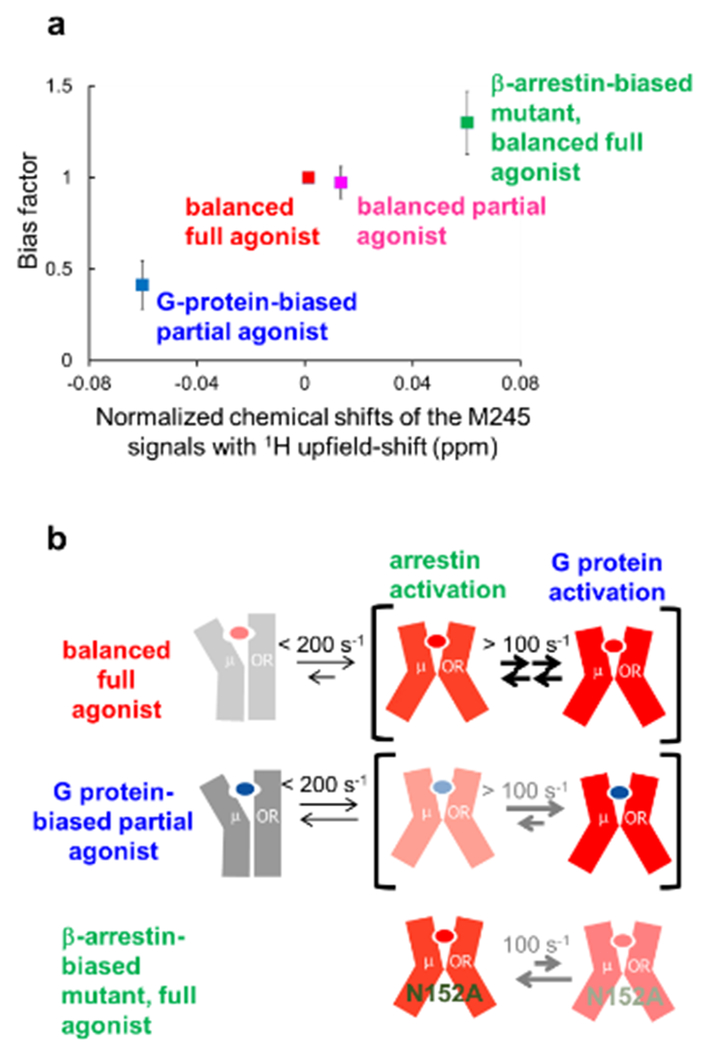
Figure 6 |. NMR-observable conformational equilibria related to biased signaling of MOR.
a | Correlation between the normalized chemical shifts of the M245 NMR signals and the value of the bias factor, which is the ratio of the β-arrestin signaling efficacy to the G protein signaling efficacy. b | Mechanisms leading to functional selectivity of MOR for different ligands. In the balanced full agonist state with DAMGO bound, MOR primarily adopts the active conformation (red), in which the intracellular surface is characterized by multiple substates, with exchange rates larger than 100 s−1. In the G protein-biased partial agonist TRV130-bound state, MOR exists in an equilibrium between the inactive (grey) and active conformations, with exchange rates smaller than 200 s−1, and the equilibrium within the active substates is shifted toward the conformation preferred for G protein activation. In the DAMGO-bound state of the MOR N1523.35A mutant, where MOR adopts only the active conformation, the equilibrium among the active substates is shifted toward the conformation preferred for arrestin activation.