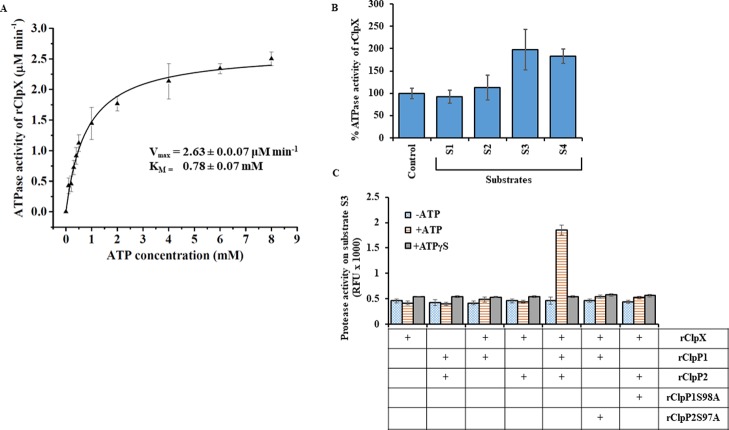
Figure 9.
Biochemical activity of purified recombinant ClpX. (A) Effect on ATPase activity of pure rClpX at increasing concentration of ATP. The rate of ATP hydrolysis by rClpX increases as a function of ATP concentration and gets saturated at 8 mM. The kinetic constants of the rClpX enzymatic reaction on ATP were determined using Michaelis–Menten kinetics. The KM or the substrate concentration corresponding to the half-maximal velocity of the reaction and maximal reaction velocity during ATP hydrolysis was determined by the nonlinear curve fitting function of Origin software. The calculated Vmax and KM of the rClpX was 2.63 ± 0.07 μM min–1 and 0.78 ± 0.07 μM, respectively. (B) The ATPase activity of pure rClpX is increased in the presence of protein substrates. Increase in ATPase activity of pure rClpX was observed in the presence of bigger protein substrates (S3 and S4) and was unaltered in the presence of small peptide substrates (S1 and S2). Substrates S3 and S4 stimulate ATPase activity of rClpX in comparison to the control where no peptide/protein substrate was present. The measured ATPase activity of rClpX for 1 h at 37 °C (control) was considered as 100%. (C) Comparison of protease activity of wild-type and mutant rClpP isoform mixture in the presence of rClpX. The wild-type rClpP isoform mixture is stimulated by rClpX to degrade fluorogenic substrate S3 only in the presence of ATP. Substitution of ATP with ATPγS, a nonhydrolysable ATP analogue, impairs substrate S3 degradation. The pure rClpP isoforms and the mutant rClpP isoform mixture fails to degrade substrate S3 in the presence of rClpX and ATP. The error bars represent the SDs from the two independent experiments performed in duplicates. Statistical analysis was performed by the Student’s t-test for comparing the rClpX ATPase activity obtained for control and in the presence of different substrates after 1.5 h of the assay (p-value <0.05).