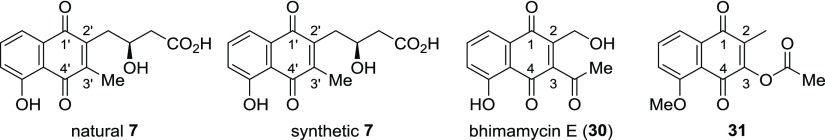
Table 1. Comparison of NMR Spectroscopic Data for Natural and Synthetic Juglomycin Z (7), Bhimamycin E (30), and 2-Methyl-3-acetoxy-5-methoxy-1,4-naphthoquinone (31).
| natural 7b |
synthetic 7c |
30d |
31e |
||||||
|---|---|---|---|---|---|---|---|---|---|
| positiona | δC | δH | δC | δH | positiona | δC | δH | δC | δH |
| 1′ | 182.2 | 184.8 | 1 | 183.1 | 185.0 | ||||
| 2′ or 3′ | 148.4 | 145.9 | 2 | 153.7 | 133.5 | ||||
| 3′ or 2′ | 149.6 | 144.2 | 3 | 119.9 | 151.9 | ||||
| 3′-Me | 18.5 | 2.67 | 12.7 | 2.26 | COMe | 30.1 | 2.34 | 20.4 | 2.06 |
| 4′ | 186.6 | 190.6 | 4 | 184.3 | 176.6 | ||||
Carbon atoms have been labeled using the IUPAC numbering system.
1H NMR (200 MHz, CDCl3/CD3OD) and 13C NMR (50.3 MHz, CDCl3).8
1H NMR (400 MHz, CDCl3/CD3OD = 9/1, TMS) and 13C NMR (100 MHz, CDCl3).
1H NMR (300 MHz, CDCl3) and 13C NMR (75.5 MHz, CDCl3).27
1H NMR (500 MHz, CDCl3) and 13C NMR (125 MHz, CDCl3, TMS).28