Abstract
Joint prosthesis failure is mainly related to aseptic loosening and prosthetic joint infections, both associated with high morbidity and a substantial cost burden for patients and health systems. The development of a biomaterial capable of stimulating bone growth while minimizing bacterial adhesion would reduce the incidence of prosthetic failure. Using an in vivo rabbit model, this study evaluates the osseointegration effect of the fluorine (F)- and phosphorus (P)-doped bottle-shaped nanostructured (bNT) Ti-6Al-4V alloy and effectiveness of monitoring urine aluminum concentration to determine the presence of Pseudomonas aeruginosa infection in Ti-6Al-4V implants. Unlike chemically polished (CP) Ti-6Al-4V alloy implants, bNT Ti-6Al-4V alloy implants promoted osseointegration and showed effectiveness as a biomaterial marker. The bNT Ti-6Al-4V alloy implants were associated with a twofold increase in bone thickness and up to 15% greater bone density compared to the CP alloy. Additionally, bNT Ti-6Al-4V alloy implants allowed for discrimination between P. aeruginosa-infected and noninfected animals for 15 days postoperatively, as indicated by the decrease of aluminum concentration in urine, while this difference was only appreciable over the first 7 days when CP Ti-6Al-4V alloy implants were used. Therefore, bNT Ti-6Al-4V alloys could have clinical applications by detecting the infection and by avoiding aseptic loosening.
1. Introduction
Joint prostheses are one of the most important medical advances in history; however, they are not free from failure; the main causes of their negative outcomes are aseptic loosening, infection, dislocation, and prosthesis or bone fracture.1
Aseptic loosening accounts for up to 30% of all surgical interventions for revision of hip and knee prostheses.2 The main causes of aseptic loosening are loss of fixation caused by inadequate initial tissue-implant fixation, mechanical loss of fixation over time, and particle-induced osteolysis around the implant.3 However, proper osseointegration may prevent aseptic loosening caused by these factors.4
Prosthetic joint infection (PJI) is an infrequent (1–2% of all cases) but dreaded complication, because of high morbidity and substantial costs which they cause.5 The most frequently isolated microorganisms from these infections are Gram-positive cocci, which represent up to 77% of all infections (mainly staphylococci).6 Gram-negative bacilli can be isolated from 3 to 45% of infections,7 and Enterobacteriaceae (mainly Escherichia coli) is the main group of species isolated,6 though nonfermenting Gram-negative bacilli such as Pseudomonas aeruginosa are gaining in importance because of the challenge they pose for treatment.7b,8 In fact, P. aeruginosa has raised special concern of late owing to its strong association with nosocomial infection and high propensity for development of antibiotic resistance.
Titanium (Ti) alloys, particularly Ti-6Al-4V, are commonly used in dental and orthopedic implants because of their favorable tribological properties and biocompatibility.9 These alloys are widely accepted to be “inert” inside the human body from the physicochemical point of view, as they are covered with a thermodynamically stable layer of titanium oxide, a continuous, highly adherent, protective, and self-repairing material. However, all metal implants undergo degradation in the human body because of at least four fundamental phenomena: leaching, wear, corrosion,10 and the synergystic phenomenon arosen from the last two,11 tribocorrosion. Corrosion and wear are the most widely studied factors in medical-use alloys. Corrosion studies of Ti-6Al-4V implants in patients have revealed elevated levels of Ti and normal levels of aluminium and vanadium in the serum or urine of carriers of a prosthesis made with this alloy, a finding which is related to aseptic loosening.12 Other studies, on the other hand, have reported that the release of Ti, aluminium, and vanadium in rabbits with implants made of this alloy may be independent of wear or loosening,11,13 with these metals reaching detectable levels even when osteosynthesis material is used.13,14 To date, no study has analyzed the possible relationship between the release of ions from the Ti-6Al-4V alloy and occurrence of PJI.
In previous studies, F-doped the Ti-6Al-4V alloy revealed that the fluorine (F)-content is the responsible for reducing staphylococcal adherence by about 50%15 and microbial biofilm development.16 Furthermore, it is known that phosphorus (P)-doped Ti alloy favors the osteointegrative properties of this alloy.17 Thus, by using together both ions, an anti-adherent and osteoinductive material could be obtained. F- and P-doped bottle-shaped nanotubular (bNT) oxide layers of Ti-6Al-4V alloy18 were obtained by the anodizing process and evaluated at microbiological and cellular levels.19 Despite bNT layers releasing aluminum (Al) from the inside of the nanostructure and the resulting ions being captured by P. aeruginosa, this biomaterial is noncytotoxic and displays improved osteoblastic proliferation, mineralization, and differentiation in vitro.19 Thus, the aim of this study was to evaluate the osseointegration of the F- and P-doped bottle-shaped nanostructured Ti-6Al-4V alloy and to monitor of urine aluminum concentration as a biomaterial marker of P. aeruginosa infection in Ti-6Al-4V implants in an in vivo rabbit model.
2. Results
2.1. Histopathologic and Microbiological Studies
The results of the histopathologic examination are shown in Table 1. The presence of nonlaminar bone was significantly superior in bNT Ti-6Al-4V implants without infection than in CP Ti-6Al-4V without infection (p = 0.0389).
Table 1. Results of Anatomopathological Studies of New-Grown Bone Samples around the Different Implants with and without P. aeruginosa Infection (Pa11) in Decalcified Samples Stained with Hematoxylin–Eosin.
| osseointegration |
osteomyelitis |
||||||
|---|---|---|---|---|---|---|---|
| material | type (n)a | >5OB/10 HPFb | nonlaminar bone | laminar bone | bone remodelation | acute | chronic |
| CP Ti-6Al-4V | NO (1) | 6 | 5 | 9 | 5 | 0 | 1 |
| PO (1) | |||||||
| CO (8) | |||||||
| CP Ti-6Al-4V + Pa11 | PO (4) | 9 | 10 | 10 | 7 | 8 | 2 |
| CO (6) | |||||||
| bNT Ti-6Al-4V | PO (4) | 10 | 10 | 10 | 9 | 0 | 0 |
| CO (6) | |||||||
| bNT Ti-6Al-4V + Pa11 | PO (3) | 10 | 10 | 10 | 7 | 7 | 3 |
| CO (7) | |||||||
NO: nonexisting osseointegration. PO: partial osseointegration. CO: complete osseointegration.
>5 osteoblasts/10 high-power fields.
Three types of bone response to the implants were observed (Figure 1): nonexisting osseointegration (Figure 1a), complete osseointegration (Figure 1b), and partial osseointegration (Figure 1c). Only one implant made of CP Ti-6Al-4V showed total absence of osseointegration (Figure 1a). The osseointegration results was obtained for the presence of five or more osteoblasts per 10 HPF; the formation of nonlamellar bone and formation of laminar bone did not allow any type of statistical inference between infected and noninfected groups (Table 1).
Figure 1.
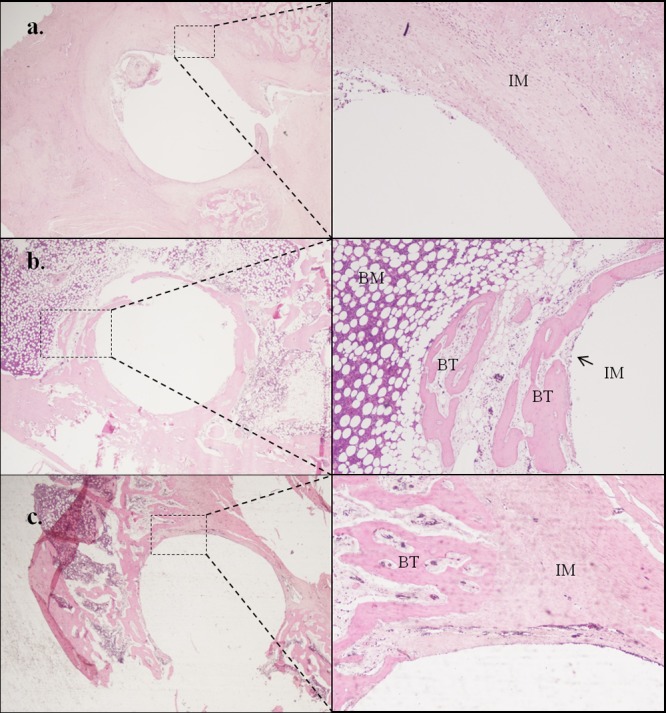
Types of osseointegration observed in the histopathologic study of decalcified periprosthetic bone samples stained with hematoxylin–eosin at 40 (left column) and 200 magnifications (right column): absence of osseointegration (a), complete osseointegration (b), and partial osseointegration (c). IM: interface membrane; BM: bone marrow; BT: bone trabecula.
Bone remodeling was significantly higher in bones placed with bNT Ti-6Al-4V implants (9/10) than in CP Ti-6Al-4V implants (5/10) (p = 0.025) (Table 1). Bone remodeling in infected joints was equal between both materials (p = 1.000).
A high rate of acute and chronic osteomyelitis was detected in infected samples with either type of implant (CP Ti-6Al-4V, 8/10 with acute osteomyelitis and 2/10 with chronic osteomyelitis; bNT Ti-6Al-4V, 7/10 with acute osteomyelitis and 3/10 with chronic osteomyelitis). The proportions of both types of osteomyelitis were equal in both materials when infection with this bacterium was induced (p = 0.303).
The bone surrounding the implants of both materials in this experimental procedure showed no signs of metallosis, neither macroscopic nor microscopic.
The results of microbiological studies showed no significant differences between implant types concerning the amount of bacteria isolated from bone and andexa on the one hand and the infected implant on the other (p > 0.05) (Figure 2). Interestingly, we observed a tendency toward greater bacteria isolation in infected bNT Ti-6Al-4V implants than in their respective bones when compared to the CP Ti-6Al-4V implants.
Figure 2.
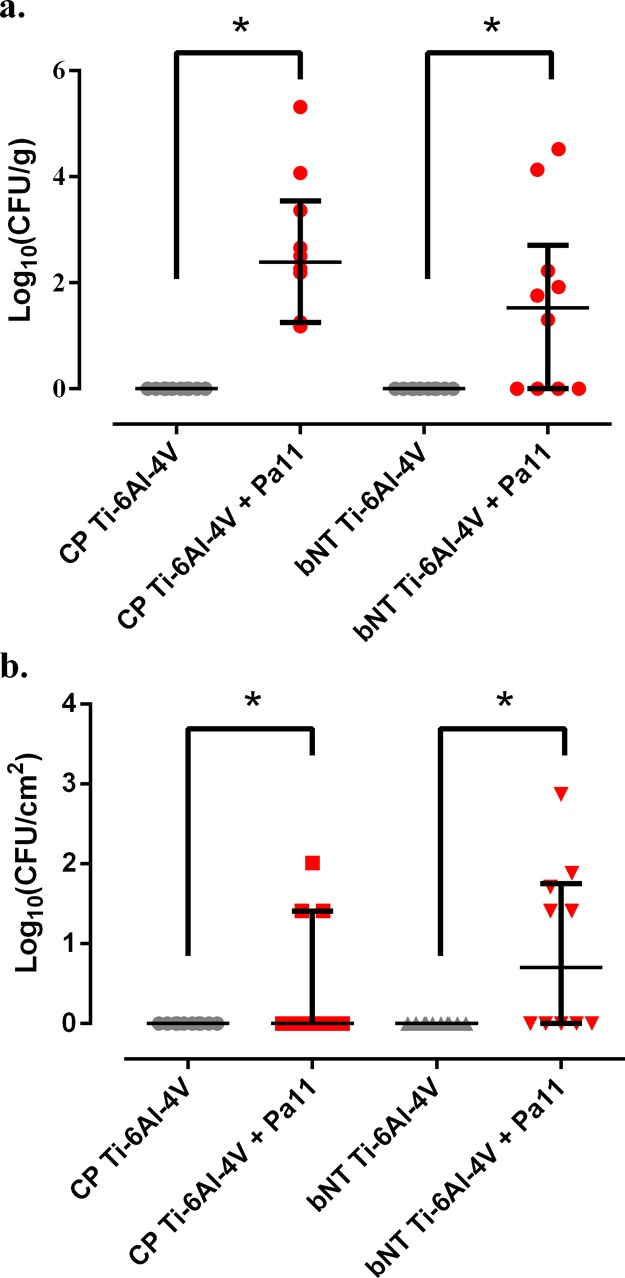
Bacterial concentration (colony-forming units, CFU) per gram of bone and adnexa (a) in the proximal femoral diaphysis and per square centimeter of implant (b) of each experimental group. The bars represent the interquartile range. *: p < 0.05 for the Wilcoxon test between the groups with P. aeruginosa infection (Pa11).
2.2. Monitoring Levels of Aluminum in Urine
The levels of Al detected in the control animals (without implant) over a 15 day monitoring period were statistically normal, and their average value (±standard deviation) was 23.13 ± 14.72 ng/mL. Over time, the mean levels of Al in urine were very similar between animals with the same type of implanted material and lower in those that had infection induced by P. aeruginosa (Figure 3a,b). The results obtained from monitoring the levels of Al in urine (ng/mL) of intervened rabbits showed that animals with Ti-6Al-4V implants had higher urine Al levels than nonintervened animals (control group) (Figure 3a,b gray area); in addition, P. aeruginosa-infected animals had lower Al levels than animals without infection. No clear trend in these levels was observed over time in either of the experimental groups. However, when a mixed-effect linear regression model was applied to the total daily Al concentration in the urine of each animal, a trend was observed in infected animals implanted with either material (p = 0.089 for CP Ti-6Al-4V and p = 0.054 for bNT Ti-6Al-4V), and a nonsignificant relation was seen between the infected animals and between noninfected animals with both materials (p > 0.05). If all Al concentrations are considered as independent observations, there were significant differences between all experimental groups (p = 0.002 for Kruskal–Wallis test) (Figure 3e), specifically between the CP Ti-6Al-4V group and CP Ti-6Al-4V + Pa11 group (p < 0.001) and between the bNT Ti-6Al-4V group and bNT Ti-6Al-4V + Pa11 group (p = 0.019). There were no significant differences between the groups without infection (p = 0.7873) or between the groups with P. aeruginosa infection (p = 0.9726). The linear mixed-effect regression model applied to the weight of the animals (Figures 3c and 4d) showed that weight gain increased over time, though without any significant difference between the experimental groups (p > 0.05). From this, it can be deduced that weight gain is not significantly different between the different experimental groups over time, which suggests that the Al coming from diet is the same in all groups and that the variations in Al levels are due only to the Al released by the implant.
Figure 3.
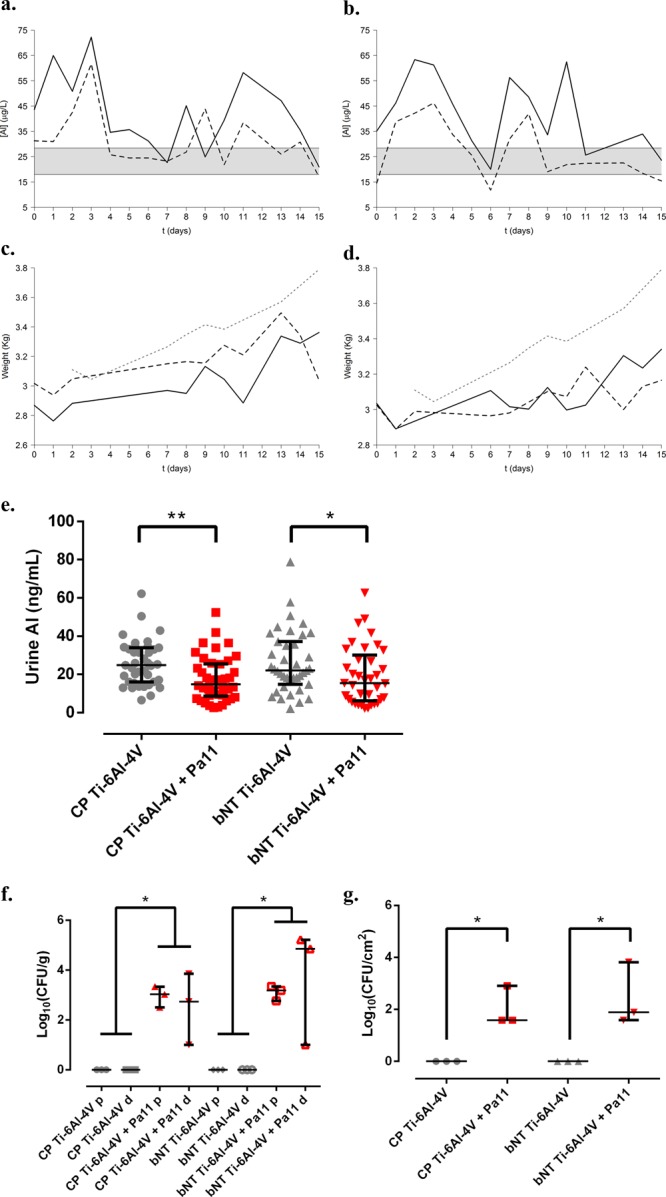
In vivo model for total Al monitoring using the urine of rabbits with a single implant with and without P. aeruginosa infection (Pa11). Mean urine Al levels and weight in rabbits with a single implant of CP Ti-6Al-4V (a,c) and bNT Ti-6Al-4V (b,d) over the first 15 days postoperatively. The black line represents the average values for animals without infection. The dotted black line represents the average values corresponding to the animals with Pa11 infection. The gray area represents the 95% confidence interval for the levels of Al in the urine of the control animals. The dotted gray line represents the average weight of the animals in the control group. Mean levels of Al in the urine of animals with an implant of each material (e). The bars represent the interquartile range. *: p < 0.05 for Wilcoxon test between the groups of each material with and without infection. **: p < 0.01 for the Wilcoxon test between groups placed with implants of each material with and without infection. Median bacterial concentration (CFU) per gram of bone and adnexa (f) in the proximal (p) and distal femoral diaphysis (d). Median bacterial concentration per square centimeter in the prostheses (g) of each experimental group. The bars represent the interquartile range. *: p < 0.05 for the Wilcoxon test between the compared groups. **: p < 0.01 for the Wilcoxon test between the compared groups.
Figure 4.
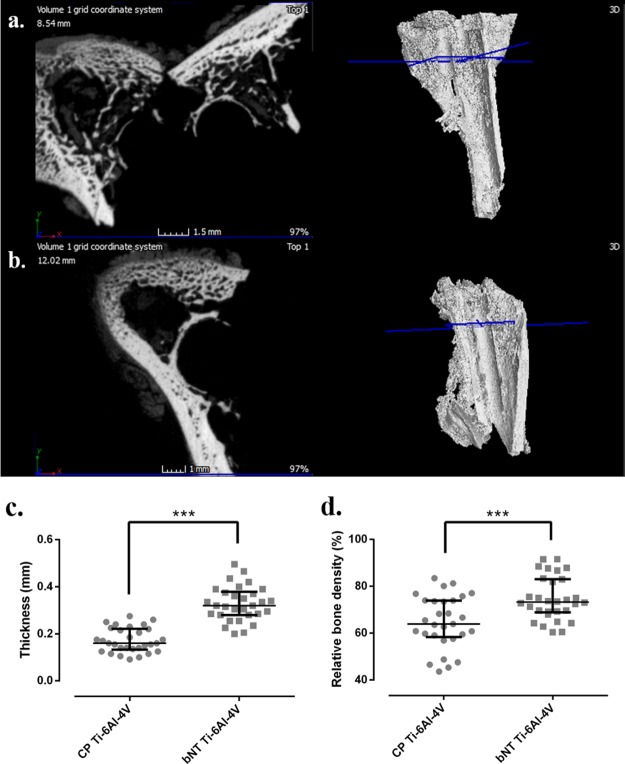
New bone grown around the CP Ti-6Al-4V implant (a), around the implant of bNT Ti-6Al-4V (b), and their respective three-dimensional representations of the femur samples by micro-CT. Results of median relative bone density (c) and thickness (d) of the new bone grown around each kind of implant. ***: p-value <0.001 for the Wilcoxon test between two materials compared.
This infection model for urine monitoring also revealed foci of osteomyelitis in the proximal femoral diaphysis, with foci of myelitis found in the distal diaphysis of two out of every three animals in which P. aeruginosa infection was induced (Figure 3f). In spite of this, there were no significant differences between implants infected with P. aeruginosa in terms of the amount of bacteria isolated from the proximal shaft (p = 0.5127) and from the distal shaft (p = 0.3758).
2.3. Computerized Microtomography Studies
The results of the computerized microtomography studies are shown in Figure 4. This figure shows the new bone grown around the CP Ti-6Al-4V implant (Figure 4a) and around the implant of bNT Ti-6Al-4V (Figure 4b), together with their respective three-dimensional views of the femur samples obtained by micro-CT. The mean new bone thickness was significantly higher in the bone grown around bNT Ti-6Al-4V than CP Ti-6Al-4V implants (p = 0.0007) (Figure 4c). Furthermore, the mean relative bone density was slightly higher in the bone grown around bNT Ti-6Al-4V than CP Ti-6Al-4V implants (p < 0.0001) (Figure 4d).
2.4. Bone Aluminum Detection
As can be seen in Figure 5, the Al detected in new growth bone was very scarce and located in the periphery of the bone and at the implant–bone interface for both kinds of implant (Figure 5a,b). The positive and negative were positive and negative, respectively (Figure 5c,d).
Figure 5.
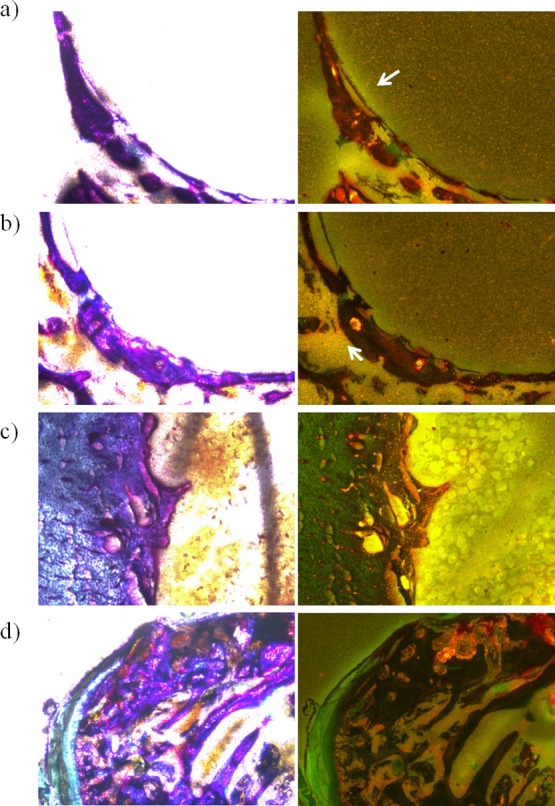
New bone grown around the CP Ti-6Al-4V implant (a) and around the implant of bNT Ti-6Al-4V (b) stained with the Walton’s aluminum stain. (c) Positive control sample. (d) Negative control sample. The white arrows point out the lightly positive area in each sample. Images of the left column are seen under white light and of the right column under ultraviolet light.
3. Discussion
In this study, we report the in vivo osseointegration ability of the F- and P-doped bNT structure. Use of the bNT Ti-6Al-4V alloy caused qualitative and quantitative improvement of the in vivo osseointegration of the implant compared to the alloy CP Ti-6Al-4V. We further show that monitoring of total Al levels in the urine of the animals used in the in vivo rabbit model during the first days postoperatively enabled discrimination between animals infected with P. aeruginosa and animals with no infection.
The bone in contact with the surface of the prosthesis undergoes morphological remodeling almost immediately after the intervention. This remodeling of mature periprosthetic bone in osseointegrated prostheses is confirmed by the presence of medullary spaces containing osteoclasts, osteoblasts, mesenchymal cells, and lymphatic/blood vessels next to the surface of the prosthesis.20 The proportion of bone samples with bone remodeling and with presence of nonlaminar around bNT Ti-6Al-4V implants was significantly higher than the proportion of bone remodeling of CP Ti-6Al-4V implants (p < 0.05). The histological results were backed up by the results of computerized microtomography studies which showed that the relative bone density and thickness of new periprosthetic bone are significantly higher (p < 0.001) in bNT Ti-6Al-4V than in CP Ti-6Al-4V, with a 15% greater relative bone density and up to twice the thickness of new periprosthetic bone (Figure 4). Thus, the F–P-doped Ti oxide layer grown onto the T-6Al-4V alloy by anodizing is a surface modification treatment that can be used to other osseointegrative treatments based on coatings such as pectin21 and nanofibrous polymers22 and other osseointegrative and anti-infective surface modifications based on graphene oxide/Ag/collagen23 and Ag/ZnO incorporated hydroxyapatite.24
P. aeruginosa has a propensity to adhere to fibro-cartilaginous and osseous joint structures and has been associated with osteomyelitis, septic arthritis, and, less frequently, PJI.8,25 The cellularity around both materials did allow a distinction to be made between acute and chronic osteomyelitis in most of the infected animals during the surgical intervention. This is the first study to highlight the release of one of the alloying elements of the Ti-6Al-4V alloy, aluminum (Al), relating this phenomenon to P. aeruginosa infection. Release of this element has been reported in different studies performed in patients with prostheses made from this alloy.11,12,14,26 The levels of Al released in patients with this type of alloy are higher in those tissues immediately adjacent to the prosthesis or implant: in descending order, these are the interface membrane, joint capsule, synovial fluid, urine, and blood.12c,27 According our results of bone Al detection, Al released from implants was lightly retained by the Ti-6Al-4V implant–bone interface membrane in both CP and bNT but not in the new growth bone (Figure 5). These findings are consistent with what other authors have affirmed.12c,27 As our experimental model is based on an intramedullary implant in the femur of each animal, when Al is released from the implant, it is incorporated into the bone through three mechanisms: heteroionic exchange with calcium, magnesium, and phosphate on the bone surface; as precipitation during bone mineralization, along with calcium and phosphate; and via coupling with nonmineralized bone tissue biomolecules, such as glycosaminoglycans, acid glycoproteins, and acidic bone matrix proteins such as phosphoproteins.28 The part of the Al that is retained on the bone surface by heteroionic exchange or binding to the organic molecules may pass into blood circulation, where it is captured by a serum and cellular component. The fraction of Al incorporated in the mineralized part of the bone is susceptible to mobilization by osteoclasts during remodeling and bone absorption.28 This mobilized Al can be excreted, mainly via the urinary system, or deposited again by any of the aforementioned mechanisms. If accurate, this physiological mechanism would justify the detection of this metal in the urine in all animal implant carriers of the Ti-6Al-4V alloy.
The Al levels detected in the control animals (without implants) can only come from water and food ingested,29 whereas the Al in the animals with a CP Ti-6Al-4V implant comes in addition to water and food from leaching, wear, corrosion,10 and/or tribocorrosion. Surface treatments allow passive layers to grow in certain metallic biomaterials that reduce the dissolution rate and, therefore, the corrosion of the metal.30 The anodizing treatment used in this work promotes the growth of an amorphous oxide layer on the surface of the alloy that increases its chemical stability and decreases corrosion, thereby lowering the levels of metal ions released into the physiological medium12c,13 while favoring the incorporation of other anions from the anodizing bath.18 However, Al was also found in the urine of the animals implanted with the bNT Ti-6Al-4V. This Al3+ is not only the product of corrosion but also the result of the release of the cation retained inside the bottle-shaped nanotubes after the anodizing process.19 The urine of P. aeruginosa-infected animals had lower Al levels than their noninfected counterparts. It had previously been shown that P. aeruginosa is capable of capturing a limited amount of Al released from bNT Ti-6Al-4V in vitro through an irreversible uptake mediated by pyochelin.19 Here, our results obtained in vivo show that this P. aeruginosa ability is maintained in the in vivo model using bNT Ti-6Al-4V but further show that the difference between P. aeruginosa infection and noninfection was also detectable when Al levels are monitored in animals with CP Ti-6Al-4V implants. Animals with CP Ti-6Al-4V implants and P. aeruginosa infection had lower Al levels during the first 7 days, whereas animals with bNT Ti-6Al-4V implants and P. aeruginosa infection showed lower levels during at least the first 15 days compared to other animals with the same material and without P. aeruginosa infection.
This experimental model has certain limitations when it comes to extrapolating the in vivo results in rabbits to humans. First, the presence or absence of infection can only be determined when the microorganism associated with PJI is P. aeruginosa. However, preliminary in vitro results suggest that other nonfermenting bacteria and certain Gram-negative fermenting species may be detected by applying this experimental model,19 although more studies are needed to corroborate these in vivo results. Second, quite often, P. aeruginosa is at least one of the two bacterial species involved in polimicrobial PJI,7b,8b,31 for that more studies will be necessary for establishing the utility of these results. Third, exposure to Al is higher and more variable in our species than in other experimental animals used because of contributing factors such as hygiene (e.g., use of cosmetics)32 and nutritional33 behaviors as well as demographic and geographic conditions. As a result, this exposure should be also considered before using this monitoring method. Fourth, bNT Ti-6Al-4V alloy may not be used in patients with kidney failure34 because Al excretion is mainly via the urinary system29a and more studies are necessary to evaluate possible Al toxicity on organs and systems (liver, kidneys, hears, nervous systems, etc). Fifth, because bNT Ti-6Al-4V roughness is almost threefold higher than that of CP Ti-6Al-4V, it cannot be exclude the possible and positive effect that roughness exert on osteointegration.35 Sixth, it should be necessary to evaluate the bNT layers using an in vivo model that allows testing biomechanically to confirm that the new bone formed around these implants is normal and actually improves the mechanical stability of the implant.
In conclusion, this is the first study to evaluate the osseostimulatory effect of the bNT Ti-6Al-4V alloy while testing the potential of urine Al concentration as a possible implant biomarker when a Ti-6Al-4V implant is infected by P. aeruginosa. Unlike similar alloys produced using CP, bNT Ti-6Al-4V both increased the relative bone density and thickness of the new bone grown around this material and also enabled discrimination between P. aeruginosa-infected and noninfected animals over the first 15 days after surgery.
4. Methods
4.1. Sample Preparation
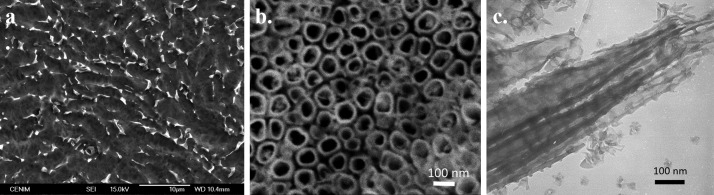
The alloy bars were prepared from 3 mm diameter Kirschner wires supplied by DePuy Synthes (Johnson & Johnson, Massachusetts, Estados Unidos). Each bar was cut into nails measuring 20 or 40 mm in length. Next, these were chemically polished (CP) according to the methodology previously described15 (Figure 6a). The roughness of the CP Ti-6Al-4V samples used ranged between 50 and 70 nm.19
Figure 6.
Scanning electronic microscopy micrograph of CP (a) and bNT Ti-6Al-4V alloy surface (b) and transmission electronic microscopy micrograph of the bNT Ti-6Al-4V nanostructure (c).
Bottle-shaped TiO2 nanotubes (bNT) were prepared by anodization according to the methodology previously described18 (Figure 6b,c). The physicochemical characterization of the bNT nanotubes has been carried out in previous studies.9b,18,36 The bNT average pore diameter are ∼47–67 nm for the mouth/bottom of the pores.18 The roughness of the bNT Ti-6Al-4V samples ranged between 150 and 170 nm.19
4.2. Surgical Procedure and Monitoring of Animals
This study was approved by the Instituto de Investigación Sanitaria de la Fundación Jiménez Díaz (IIS-FJD) Animal Care and Use Committee, which includes ad hoc members for ethical issues. Animal care and maintenance complied with institutional guidelines as defined in national and international laws and policies (Spanish Royal Decree 53/2013, authorization reference PROEX111/16 December 28, 2016, by the Ministry of the Environment, Local Administration and Territorial Planning of the Community of Madrid and, Directive 2010/63/EU of the European Parliament and of the Council of September 22, 2010).
Specific pathogen-free New Zealand white male rabbits (Granja San Bernardo, Navarra, Spain) between 2.5 and 3 kg of weight were used. All animals were individually housed in individual cages in an air-conditioned room at 22 ± 2 °C and cycles of light-darkness of 12:12 h.
In this in vivo model, we used a P. aeruginosa strain isolated from an 80 year-old woman with an infection of spinal osteosynthesis material (Pa11) in the Clinical Microbiology department of the Fundación Jiménez Díaz University Hospital.
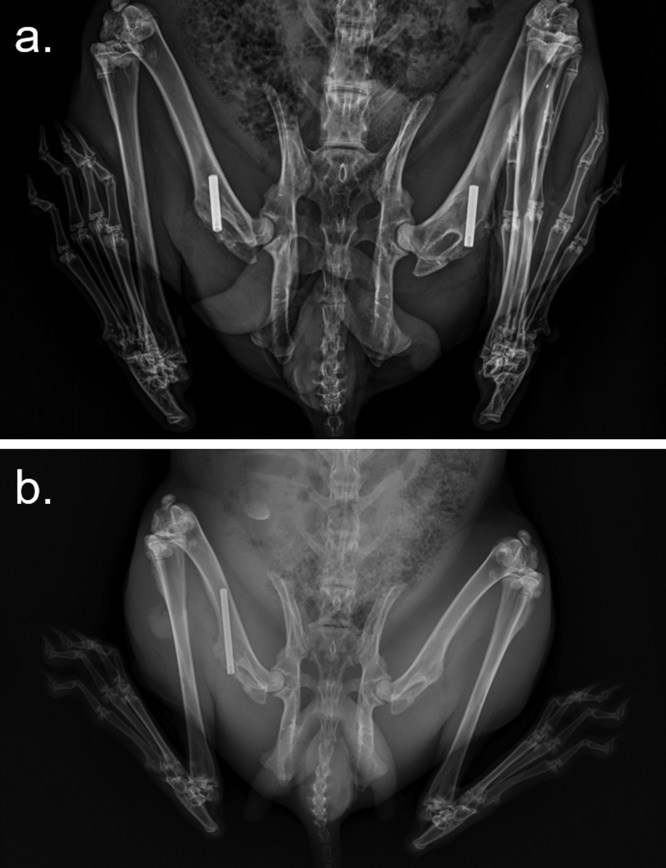
The surgical intervention of the in vivo model was based on the model previously described by Cordero et al.37 Above the drilled hole, 100 μL of 0.9% NaCl physiological saline was injected with or without a total concentration of 106 CFU of bacteria obtained from Pa11. Subsequently, each cylinder of Ti-6Al-4V was implanted in accordance with the group to which each animal was assigned until the implant was flushed with the joint surface. To reduce the number of animals used, both femurs of each animal were intervened, leaving 1 week between operations because animals do not tolerate implantation well in both femurs in a single surgery (Figure 7a). Each animal received two implants of the same kind of material and same treatment. No differences were detected between both legs despite the temporal difference, in a similar way to other authors.21,37
Figure 7.
Radiographies of the surgery rabbit models in this work: 20 mm long implant and one implant per femur (a) and 40 mm long implant and one implant per animal (b).
We used 20 rabbits with femoral implants distributed into four groups: one group with a CP Ti-6Al-4V implant without infection (CP Ti-6Al-4V group), a second group with a CP Ti-6Al-4V implant with infection induced by Pa11 P. aeruginosa (CP Ti-6Al-4V + Pa11 group), third group with an implant of bNT Ti-6Al-4V without infection (bNT Ti-6Al-4V group), and a fourth group having an implant of bNT Ti-6Al-4V with infection induced by P. aeruginosa (bNT Ti-6Al-4V + Pa11 group).
Four weeks after the second surgery, each animal was euthanized under general anesthesia by intracardiac overdose of sodium thiobarbital. The rabbit femurs were recovered through sterile preparation of the hip, surgical field isolation, and by following the approach described for the primary surgery.
4.3. Pathologic and Microbiological Processing of Samples
The periprosthetic bone was longitudinally divided into two samples using chisel and hammer on a sterile surface per sample.
Histological sections were fixed, decalcificated, paraffin-infiltrated, and hematoxylin–eosin stained. The osseointegration reaction was evaluated by classifying the specimens as complete, partial, or nonexistent osseointegration. Complete osseointegration was established as the formation of a complete structure of bony trabeculae around the implant, whether from woven-immature or mature-laminar bone, with scant interface membrane remaining. Partial osseointegration was defined as focal bone formation with unossified areas, with the interface membrane still occupying half of the periprosthetic tissue, whereas the absence of osseointegration was taken to be the absence of bone formation, with all periprosthetic tissue being the interface membrane. Bone remodeling was evaluated in a binary manner based on the presence of remodeling cellularity (osteoblasts and osteoclasts) in the newly formed trabeculae closest to the implant and the presence of laminar or nonlaminar bone. Within osteomyelitis, a distinction was made between acute and chronic infection in the entire bone according to the criterion previously described.38
For microbiological studies, the bone and the implant were separately immersed in phosphate-buffered saline (Biomérieux, Marcy-l’Étoile, France) and sonicated using an Ultrasons-H 3000840 low-power bath sonicator (J.P. Selecta, Barcelona, Spain) at 22 °C for 5 min.39 The resulting sonicate was diluted in a 10-fold dilution bank and seeded on blood-chocolate agar (Biomérieux, Marcy-l’Étoile, France) using the spread plate method. The concentration of bacteria was estimated as CFU/g of bone and adnexa or as CFU/cm2 of implant.
4.4. Monitoring the Levels of Aluminum in Urine
For this study, the same experimental procedure previously described was performed, albeit with the modifications described below. For better monitoring of Al levels in the urine of the animals, some rabbits underwent surgery only once, using 40 mm long nails of each material and were infected with a bacterial inoculum of 200 μL of solution with a 107 CFU/mL concentration of Pa11 (Figure 7b). At least 1 mL of urine per animal was collected by means of nonforced urination from day 0 until the day of euthanasia.
Total Al concentration in the urine was estimated by atomic adsorption in a graphite furnace at Reference Laboratory (Barcelona, Spain). We used 14 animals placed with femur implants and distributed them into five groups (including the above-mentioned ones): CP Ti-6Al-4V group, CP Ti-6Al-4V + Pa11 group, bNT Ti-6Al-4V group, bNT Ti-6Al-4V + Pa11 group, and a fifth group composed of two animals without an implant and without infection, used as controls to establish baseline levels of Al in the rabbits’ urine. The entire femur was removed from each animal, and the proximal and distal femoral diaphyses were analyzed separately at pathological and microbiological level.
4.5. Computerized Microtomography Studies
The fixed samples were scanned with an in vitro micro-CT device (Nikon 160 XTH). The scanning parameters were as follows: 139 kV, 52 μA, 1000 projections, exposure 708 ms/frame, average of 4 frames per projection, and a 0.25 mm copper filter with 1.5 h of scanning time. X-ray projection reconstructions were made with MyVGL (see 3.1 Volume Graphics, Heidelberg, Germany). Two parameters were estimated with this software: mean thickness of new bone and mean relative bone density. Mean new bone thickness was estimated from two measures on the same plane of the new bone grown around the implant (10 measures on 10 planes covering at least 1 cm per bone sample). The mean relative bone density was estimated from the coefficient of the mean of three measures of gray level in the peri-implant new bone and mean of three measures of denser cortical bone gray shade from each plane (10 measures of new-grown bone closest to the implant on 10 different CT slides chosen at random covering at least 1 cm per bone sample).
4.6. Aluminum Detection in Bone
One fixed sample of bone from each group, the CP Ti-6Al-4V alloy, and bNT Ti-6Al-4V alloy were included in the EpoFix resin Kit (Struers ApS, Ballerup, Denmark), cut, and sanded to a thickness of 30–40 μm. The presence of Al in bone was determined using the staining procedure described by Walton et al.40 The Walton histological method stains aluminum that is covalently bound to tissue components but also forms a complex with the carboxylate group of the phoxine dye, which leads to a stable intracellular magenta-colored appearance visible to the optical microscope.40 Under ultraviolet light, the sensitivity of the method improves further, and the stained aluminium at the surface can be easily discriminated from other stained features.40 The positive control was performed using a rabbit femur drilled and injected with 200 μL of 1 mg/mL of AlCl3 in SS without an implant.
4.7. Statistical
Statistical analyses were performed using Stata Statistical Software, Release 11 (StataCorp 2009). First, the normality of each series of data was checked with the Shapiro–Wilk test.
Pathological results were evaluated using the comparation of two proportions between groups.
Microbiological and computerized micrography results were evaluated using the one-sided Wilcoxon nonparametric test that was applied to compare two groups of data, while the Kruskal–Wallis test was used when there were more than two groups.
We compared the Al in the urine of each animal each day, and weight gain was compared through a unilateral mixed-effect regression model. In each experimental group, the rate of excretion was estimated every 24 h by calculating the product of the concentration of Al (ng/mL), the average amount of urine (62.5 ± 12.5 mL/kg),29b and the average weight of each group per day.
A level of statistical significance of p ≤ 0.05 was considered significant. All data are represented as mean and standard deviation for statistically normal results and as median and interquartile range for statistically non-normal results.
Acknowledgments
This study was funded by a grant from the Spanish Ministry of Economics and Competitiveness (MAT2017-86163-C2-1-R, MAT2013-48224-C2-2-R, and MAT2013-48224-C2-1-R). J.-J.A.C. is funded by an FPI grant from the same institution (BES-2014-069007). We wish to acknowledge Dr. Oliver Shaw for his help in revising the manuscript for aspects related to the English language. Part of this work was previously presented before the 28th European Congress of Clinical Microbiology and Infectious Diseases.
Author Contributions
J.-J.A.-C., A.A., M.B.-S., I.M.-F., A.M., D.E.-B., A.C., M.-A.A., J.-J.d.-D., J.C.A., and J.E. made substantial contributions to research design, or the acquisition, analysis, or interpretation of data; J.-J.A.-C., A.C., M.-A.A., J.-J.d.D., and J.E. drafted the paper or revised it critically; J.-J.A.-C., A.C., M.-A.A., J.-J.d.D., J.C.-A., and J.E. gained approval of the submitted and final versions. All authors have read and approved this final submitted manuscript.
The authors declare no competing financial interest.
References
- Del Pozo J. L.; Patel R. Infection Associated with Prosthetic Joints. N. Engl. J. Med. 2009, 361, 787–794. 10.1056/nejmcp0905029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Khan M.; Osman K.; Green G.; Haddad F. S. The epidemiology of failure in total knee arthroplasty. Bone Jt. J. 2016, 98-B, 105–112. 10.1302/0301-620x.98b1.36293. [DOI] [PubMed] [Google Scholar]; b Springer B. D.; Fehring T. K.; Griffin W. L.; Odum S. M.; Masonis J. L. Why revision total hip arthroplasty fails. Clin. Orthop. Relat. Res. 2009, 467, 166–173. 10.1007/s11999-008-0566-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu-Amer Y.; Darwech I.; Clohisy J. C. Aseptic loosening of total joint replacements: mechanisms underlying osteolysis and potential therapies. Arthritis Res. Ther. 2007, 9, S6. 10.1186/ar2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piscitelli P.; Iolascon G.; Innocenti M.; Civinini R.; Rubinacci A.; Muratore M.; D’Arienzo M.; Leali P. T.; Carossino A. M.; Brandi M. L. Painful prosthesis: approaching the patient with persistent pain following total hip and knee arthroplasty. Clin. Cases Miner. Bone Metab. 2013, 10, 97–110. 10.11138/ccmbm/2013.10.2.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tande A. J.; Osmon D. R.; Greenwood-Quaintance K. E.; Mabry T. M.; Hanssen A. D.; Patel R. Clinical characteristics and outcomes of prosthetic joint infection caused by small colony variant staphylococci. mBio 2014, 5, e01910–e01914. 10.1128/mbio.01910-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito N.; Franco M.; Ribera A.; Soriano A.; Rodriguez-Pardo D.; Sorli L.; Fresco G.; Fernandez-Sampedro M.; Dolores Del Toro M.; Guio L.; Sanchez-Rivas E.; Bahamonde A.; Riera M.; Esteban J.; Baraia-Etxaburu J. M.; Martinez-Alvarez J.; Jover-Saenz A.; Duenas C.; Ramos A.; Sobrino B.; Euba G.; Morata L.; Pigrau C.; Coll P.; Mur I.; Ariza J. Time trends in the aetiology of prosthetic joint infections: a multicentre cohort study. Clin. Microbiol. Infect. 2016, 22, 732e1–732e8. 10.1016/j.cmi.2016.05.004. [DOI] [PubMed] [Google Scholar]
- a Cobo J.; Miguel L. G. S.; Euba G.; Rodríguez D.; García-Lechuz J. M.; Riera M.; Falgueras L.; Palomino J.; Benito N.; del Toro M. D.; Pigrau C.; Ariza J. Early prosthetic joint infection: outcomes with debridement and implant retention followed by antibiotic therapy. Clin. Microbiol. Infect. 2011, 17, 1632–1637. 10.1111/j.1469-0691.2010.03333.x. [DOI] [PubMed] [Google Scholar]; b Rodríguez-Pardo D.; Pigrau C.; Lora-Tamayo J.; Soriano A.; del Toro M. D.; Cobo J.; Palomino J.; Euba G.; Riera M.; Sánchez-Somolinos M.; Benito N.; Fernández-Sampedro M.; Sorli L.; Guio L.; Iribarren J. A.; Baraia-Etxaburu J. M.; Ramos A.; Bahamonde A.; Flores-Sánchez X.; Corona P. S.; Ariza J. Gram-negative prosthetic joint infection: outcome of a debridement, antibiotics and implant retention approach. A large multicentre study. Clin. Microbiol. Infect. 2014, 20, O911–O919. 10.1111/1469-0691.12649. [DOI] [PubMed] [Google Scholar]
- a Hsieh P. H.; Lee M. S.; Hsu K.-Y.; Chang Y.-H.; Shih H.-N.; Ueng S. W. Gram-Negative Prosthetic Joint Infections: Risk Factors and Outcome of Treatment. Clin. Infect. Dis. 2009, 49, 1036–1043. 10.1086/605593. [DOI] [PubMed] [Google Scholar]; b Shah N. B.; Osmon D. R.; Steckelberg J. M.; Sierra R. J.; Walker R. C.; Tande A. J.; Berbari E. F. Pseudomonas Prosthetic Joint Infections: A Review of 102 Episodes. J Bone Jt. Infect. 2016, 1, 25–30. 10.7150/jbji.15722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Golvano I.; Garcia I.; Conde A.; Tato W.; Aginagalde A. Influence of fluoride content and pH on corrosion and tribocorrosion behaviour of Ti13Nb13Zr alloy in oral environment. J. Mech. Behav. Biomed. Mater. 2015, 49, 186–196. 10.1016/j.jmbbm.2015.05.008. [DOI] [PubMed] [Google Scholar]; b Hernández-López J. M.; Conde A.; de Damborenea J.; Arenas M. A. Correlation of the nanostructure of the anodic layers fabricated on Ti13Nb13Zr with the electrochemical impedance response. Corros. Sci. 2015, 94, 61–69. 10.1016/j.corsci.2015.01.041. [DOI] [Google Scholar]; c Long M.; Rack H. J. Titanium alloys in total joint replacement-a materials science perspective. Biomaterials 1998, 19, 1621–1639. 10.1016/s0142-9612(97)00146-4. [DOI] [PubMed] [Google Scholar]
- a Soto-Alvaredo J.; Blanco E.; Bettmer J.; Hevia D.; Sainz R. M.; López Cháves C.; Sánchez C.; Llopis J.; Sanz-Medel A.; Montes-Bayón M. Evaluation of the biological effect of Ti generated debris from metal implants: ions and nanoparticles. Metallomics 2014, 6, 1702–1708. 10.1039/c4mt00133h. [DOI] [PubMed] [Google Scholar]; b Wolford L. M. Factors to consider in joint prosthesis systems. Proc.—Bayl. Univ. Med. Cent. 2006, 19, 232–238. 10.1080/08998280.2006.11928170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J. J.; Gilbert J. L.; Urban R. M. Corrosion of Metal Orthopaedic Implants*. J. Bone Jt. Surg., Am. Vol. 1998, 80, 268–282. 10.2106/00004623-199802000-00015. [DOI] [PubMed] [Google Scholar]
- a Jacobs J. J.; Silverton C.; Hallab N. J.; Skipor A. K.; Patterson L.; Black J.; Galante J. O. Metal release and excretion from cementless titanium alloy total knee replacements. Clin. Orthop. Relat. Res. 1999, 358, 173–180. 10.1097/00003086-199901000-00021. [DOI] [PubMed] [Google Scholar]; b Jacobs J. J.; Skipor A. K.; Black J.; Urban R.; Galante J. O. Release and excretion of metal in patients who have a total hip-replacement component made of titanium-base alloy. J. Bone Jt. Surg., Am. Vol. 1991, 73, 1475–1486. 10.2106/00004623-199173100-00005. [DOI] [PubMed] [Google Scholar]; c Jacobs J. J.; Skipor A. K.; Patterson L. M.; Hallab N. J.; Paprosky W. G.; Black J.; Galante J. O. Metal release in patients who have had a primary total hip arthroplasty. A prospective, controlled, longitudinal study. J. Bone Jt. Surg., Am. Vol. 1998, 80, 1447–1458. 10.2106/00004623-199810000-00006. [DOI] [PubMed] [Google Scholar]
- Bianco P. D.; Ducheyne P.; Cuckler J. M. Titanium serum and urine levels in rabbits with a titanium implant in the absence of wear. Biomaterials 1996, 17, 1937–1942. 10.1016/0142-9612(96)00023-3. [DOI] [PubMed] [Google Scholar]
- Nuevo-Ordóñez Y.; Montes-Bayón M.; Blanco-González E.; Paz-Aparicio J.; Raimundez J. D.; Tejerina J. M.; Peña M. A.; Sanz-Medel A. Titanium release in serum of patients with different bone fixation implants and its interaction with serum biomolecules at physiological levels. Anal. Bioanal. Chem. 2011, 401, 2747–2754. 10.1007/s00216-011-5232-8. [DOI] [PubMed] [Google Scholar]
- Arenas M. A.; Pérez-Jorge C.; Conde A.; Matykina E.; Hernández-López J. M.; Pérez-Tanoira R.; de Damborenea J. J.; Gómez-Barrena E.; Esteba J. Doped TiO2 anodic layers of enhanced antibacterial properties. Colloids Surf., B 2013, 105, 106–112. 10.1016/j.colsurfb.2012.12.051. [DOI] [PubMed] [Google Scholar]
- Perez-Jorge C.; Arenas M.-A.; Conde A.; Hernandez-Lopez J.-M.; de Damborenea J.-J.; Fisher S.; Hunt A. M.; Esteban J.; James G. Bacterial and fungal biofilm formation on anodized titanium alloys with fluorine. J. Mater. Sci.: Mater. Med. 2017, 28, 8. 10.1007/s10856-016-5811-5. [DOI] [PubMed] [Google Scholar]
- Katona B.; Dobos G.; Kiss G. Examination of the Surface Phosphorus Content of Anodized Medical Grade Titanium Samples. Mater. Sci. Forum 2015, 812, 339–344. 10.4028/www.scientific.net/msf.812.339. [DOI] [Google Scholar]
- Hernández-López J. M.; Conde A.; de Damborenea J. J.; Arenas M. A. TiO2nanotubes with tunable morphologies. RSC Adv. 2014, 4, 62576–62585. 10.1039/c4ra11457d. [DOI] [Google Scholar]
- Aguilera-Correa J.-J.; Mediero A.; Conesa-Buendia F.-M.; Conde A.; Arenas M.-A.; de-Damborenea J.-J.; Esteban J. Microbiological and cellular evaluation of fluorine-phosphorus doped titanium alloy: A novel antibacterial and osteostimulatory biomaterial with potential applications in orthopedic surgery. Appl. Environ. Microbiol. 2018, 85, e02271. 10.1128/aem.02271-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavrogenis A. F.; Dimitriou R.; Parvizi J.; Babis G. C. Biology of implant osseointegration. J. Musculoskeletal Neuronal Interact. 2009, 9, 61–71. [PubMed] [Google Scholar]
- Gurzawska K.; Dirscherl K.; Jørgensen B.; Berglundh T.; Jørgensen N. R.; Gotfredsen K. Pectin nanocoating of titanium implant surfaces - an experimental study in rabbits. Clin. Oral Implant. Res. 2017, 28, 298–307. 10.1111/clr.12798. [DOI] [PubMed] [Google Scholar]
- Das S.; Gurav S.; Soni V.; Ingle A.; Mohanty B. S.; Chaudhari P.; Bendale K.; Dholam K.; Bellare J. R. Osteogenic Nanofibrous Coated Titanium Implant Results in Enhanced Osseointegration: In Vivo Preliminary Study in a Rabbit Model. Tissue Eng. Regener. Med. 2018, 15, 231–247. 10.1007/s13770-017-0106-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X.; Mao C.; Liu X.; Zhang Y.; Cui Z.; Yang X.; Yeung K. W. K.; Pan H.; Chu P. K.; Wu S. Synergistic Bacteria Killing through Photodynamic and Physical Actions of Graphene Oxide/Ag/Collagen Coating. ACS Appl. Mater. Interfaces 2017, 9, 26417–26428. 10.1021/acsami.7b06702. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Liu X.; Li Z.; Zhu S.; Yuan X.; Cui Z.; Yang X.; Chu P. K.; Wu S. Nano Ag/ZnO-Incorporated Hydroxyapatite Composite Coatings: Highly Effective Infection Prevention and Excellent Osteointegration. ACS Appl. Mater. Interfaces 2018, 10, 1266–1277. 10.1021/acsami.7b17351. [DOI] [PubMed] [Google Scholar]
- Martinez-Pastor J. C.; Munoz-Mahamud E.; Vilchez F.; Garcia-Ramiro S.; Bori G.; Sierra J.; Martinez J. A.; Font L.; Mensa J.; Soriano A. Outcome of acute prosthetic joint infections due to gram-negative bacilli treated with open debridement and retention of the prosthesis. Antimicrob. Agents Chemother. 2009, 53, 4772–4777. 10.1128/aac.00188-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton M. S.; Lyon T. D. B.; Ashcroft G. P. Levels of systemic metal ions in patients with intramedullary nails. Acta Orthop. 2008, 79, 820–825. 10.1080/17453670810016911. [DOI] [PubMed] [Google Scholar]
- Sargeant A.; Goswami T.; Swank M. Ion concentrations from hip implants. J. Surg. Orthop. Adv. 2006, 15, 113–114. [PubMed] [Google Scholar]
- Priest N. D. The biological behaviour and bioavailability of aluminium in man, with special reference to studies employing aluminium-26 as a tracer: review and study update. J Environ Monit 2004, 6, 375–403. 10.1039/b314329p. [DOI] [PubMed] [Google Scholar]
- a Exley C. Human exposure to aluminium. Environ. Sci. Process. Impacts 2013, 15, 1807–1816. 10.1039/c3em00374d. [DOI] [PubMed] [Google Scholar]; b Suckow M. A.; Schroeder V.; Douglas F. A.. The Laboratory Rabbit, 2nd ed.; CRC Press, 2010. [Google Scholar]
- Bhola R.; Bhola S. M.; Mishra B.; Olson D. L. Corrosion in titanium dental implants/prostheses–a review. Trends Biomater. Artif. Organs 2011, 25, 34–46. [Google Scholar]
- Marculescu C. E.; Cantey J. R. Polymicrobial prosthetic joint infections: risk factors and outcome. Clin. Orthop. Relat. Res. 2008, 466, 1397–1404. 10.1007/s11999-008-0230-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineau A.; Fauconneau B.; Sappino A.-P.; Deloncle R.; Guillard O. If exposure to aluminium in antiperspirants presents health risks, its content should be reduced. J. Trace Elem. Med. Biol. 2014, 28, 147–150. 10.1016/j.jtemb.2013.12.002. [DOI] [PubMed] [Google Scholar]
- Greger J. L. Dietary and other sources of aluminium intake. Ciba Found. Symp. 1992, 169, 26–49. ; discussion 35–49 10.1002/9780470514306.ch3. [DOI] [PubMed] [Google Scholar]
- a Robertson J. A.; Felsenfeld A. J.; Haygood C. C.; Wilson P.; Clarke C.; Llach F. Animal model of aluminum-induced osteomalacia: role of chronic renal failure. Kidney Int. 1983, 23, 327–335. 10.1038/ki.1983.23. [DOI] [PubMed] [Google Scholar]; b Wills M. R.; Savory J. Water content of aluminum, dialysis dementia, and osteomalacia. Environ. Health Perspect. 1985, 63, 141–147. 10.1289/ehp.8563141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L.; Wang H.; Ni M.; Rui Y.; Cheng T.-Y.; Cheng C.-K.; Pan X.; Li G.; Lin C. Enhanced osteointegration of medical titanium implant with surface modifications in micro/nanoscale structures. J. Orthop. Translat. 2014, 2, 35–42. 10.1016/j.jot.2013.08.001. [DOI] [Google Scholar]
- Matykina E.; Hernandez-López J. M.; Conde A.; Domingo C.; de Damborenea J. J.; Arenas M. A. Morphologies of nanostructured TiO2 doped with F on Ti-6Al-4V alloy. Electrochim. Acta 2011, 56, 2221–2229. 10.1016/j.electacta.2010.11.069. [DOI] [Google Scholar]
- Cordera J.; Munuera L.; Folgueira M. D. Influence of bacterial strains on bone infection. J. Orthop. Res. 1996, 14, 663–667. 10.1002/jor.1100140423. [DOI] [PubMed] [Google Scholar]
- Petty W.; Spanier S.; Shuster J. J.; Silverthorne C. The influence of skeletal implants on incidence of infection. Experiments in a canine model. J. Bone Jt. Surg., Am. Vol. 1985, 67, 1236–1244. 10.2106/00004623-198567080-00015. [DOI] [PubMed] [Google Scholar]
- Esteban J.; Gomez-Barrena E.; Cordero J.; Martin-de-Hijas N. Z.; Kinnari T. J.; Fernandez-Roblas R. Evaluation of quantitative analysis of cultures from sonicated retrieved orthopedic implants in diagnosis of orthopedic infection. J. Clin. Microbiol. 2008, 46, 488–492. 10.1128/jcm.01762-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton J. R.; Diamond T. H.; Kumar S.; Murrell G. A. C. A sensitive stain for aluminum in undecalcified cancellous bone. J. Inorg. Biochem. 2007, 101, 1285–1290. 10.1016/j.jinorgbio.2007.06.006. [DOI] [PubMed] [Google Scholar]