Abstract
Background
Radiographic leptomeningeal disease (LMD) develops in up to 30% of patients following postoperative stereotactic radiosurgery (SRS) for brain metastases. However, the clinical relevancy of this finding and outcomes after various salvage treatments are not known.
Methods
Patients with brain metastases, of which 1 was resected and treated with adjunctive SRS, and who subsequently developed LMD were combined from 7 tertiary care centers. LMD pattern was categorized as nodular (nLMD) or classical (“sugarcoating,” cLMD).
Results
The study cohort was 147 patients. Most patients (60%) were symptomatic at LMD presentation, with cLMD more likely to be symptomatic than nLMD (71% vs. 51%, P = 0.01). Salvage therapy was whole brain radiotherapy (WBRT) alone (47%), SRS (27%), craniospinal radiotherapy (RT) (10%), and other (16%), with 58% receiving a WBRT-containing regimen. WBRT was associated with lower second LMD recurrence compared with focal RT (40% vs 68%, P = 0.02). Patients with nLMD had longer median overall survival (OS) than those with cLMD (8.2 vs 3.3 mo, P < 0.001). On multivariable analysis for OS, pattern of initial LMD (nodular vs classical) was significant, but type of salvage RT (WBRT vs focal) was not.
Conclusions
Nodular LMD is a distinct pattern of LMD associated with postoperative SRS that is less likely to be symptomatic and has better OS outcomes than classical “sugarcoating” LMD. Although focal RT demonstrated increased second LMD recurrence compared with WBRT, there was no associated OS detriment. Focal cranial RT for nLMD recurrence after surgery and SRS for brain metastases may be a reasonable alternative to WBRT.
Keywords: radiosurgery, brain metastases, leptomeningeal disease, postoperative radiosurgery
Most patients with radiographic LMD after surgery and SRS for brain metastases are symptomatic.
Patients with the nodular pattern of LMD recurrence were less symptomatic and had longer median OS compared with the classical sugarcoating LMD pattern.
Focal cranial RT for nodular LMD was associated with higher risk of second LMD recurrence compared with WBRT, but without a detriment in OS.
Importance of the Study.
Leptomeningeal disease occurs in up to 30% of patients treated with surgery and adjunctive SRS for brain metastases. The clinical relevance of radiographic LMD, the specific patterns of recurrence, and patient outcomes based on LMD management in this clinical setting have not been previously reported. We demonstrated that radiographic LMD is clinically significant in terms of symptom burden at presentation. Additionally, we describe novel findings that nodular pattern LMD may have a distinct biological behavior from classical “sugarcoating” LMD with less symptoms at presentation and significantly better OS expectations regardless of the type of cranial RT treatment for LMD. Patients with nodular LMD treated with focal cranial RT had increased second LMD recurrence, but no OS detriment compared with WBRT-containing regimens. Based on these findings, focal cranial RT for nodular LMD recurrence after surgery and SRS for brain metastases may be a reasonable alternative to WBRT.
Up to 30% of patients with solid cancer will develop brain metastases (BM), which represent a significant source of morbidity and mortality.1 The incidence of BM has increased in recent years, possibly due to improved sensitivity of neuroimaging, improved systemic therapies with limited brain penetration, or a combination of factors.2
Stereotactic radiosurgery (SRS) for patients with a limited number of brain metastases (defined as 1–4) is a standard of care based on multiple phase III randomized trials demonstrating no detriment in overall survival (OS) and reduced risk of neurocognitive and quality of life deterioration with the omission of whole brain radiotherapy (WBRT), albeit with increased risk of intracranial failure.3–7 Surgical resection of BM is associated with a 1- to 2-year risk of cavity local recurrence (LR) of approximately 47–59%, hence adjuvant cranial RT is generally offered in the United States to minimize risk of cavity LR after resection of BM.6,8–10 Recently, 2 phase III randomized trials were published which demonstrated improved cavity local control for postop SRS compared with resection alone and improvement in neurocognitive preservation compared with WBRT, establishing postop SRS as a standard of care.9,11
However, postop SRS has also been associated with increased rates of leptomeningeal disease (LMD) recurrence, with reported incidence of up to 31% at 1 year (Supplementary Table 1).9,12 Several studies have demonstrated that surgical resection of BM is associated with significantly higher rates of LMD compared with intact BM treated with SRS.13–15 Other risk factors for LMD in the postoperative setting include breast cancer histology, piecemeal resection of BM, posterior fossa location, multiple BM, and hemorrhagic or cystic features.12,16–20 It is thought that this increased risk is due to tumor spillage into the cerebrospinal fluid (CSF) at the time of surgical resection, which was not as clinically apparent or relevant when the entire intracranial CSF space was treated with routine postop WBRT.21 In addition, a unique pattern of LMD failure consisting of only focal or nodular meningeal enhancement has been described in the postop SRS setting, but the clinical relevance of this finding has not been well described.12,19,22
The specifics of the radiographic and clinical findings and prognostic significance of LMD recurrence in this setting are not well characterized. The purpose of this study was to determine the presentation, patterns of failure, and outcomes based on management for patients with BM treated with resection and adjunctive SRS who developed LMD recurrence.
Materials and Methods
Patients
Patients with BM from solid tumors, of which 1 was resected and treated with adjunctive SRS (either preop or postop), and who developed subsequent LMD were included. Patient information was gathered from 7 tertiary care centers under separate institutional review board–approved protocols from either prospectively or retrospectively maintained institutional databases. Institutional review board waivers of informed consent were obtained due to the minimal patient risk associated with observational databases. Exclusion criteria included classically radiosensitive tumors (eg, small-cell cancer, lymphoma, germ cell tumor), previous WBRT, planned adjuvant WBRT, or more than 1 resected BM.
Treatments
The decision for surgical resection of the BM was made at each respective center. SRS was delivered using either Gamma Knife (Elekta), CyberKnife (Accuray), or LINAC (linear accelerator) platforms. Postop SRS was performed according to standard of care specific to the treating institution and platform used. In general, postop SRS was delivered 2–4 weeks after surgical resection to the cavity with a 0–2 mm margin in 1–5 fractions, depending on cavity volume. Preop SRS was delivered in a single fraction without margin expansion with an approximate 10–20% dose reduction compared with standard lesion size–based dosing, as previously described.17,23–26 Surgery generally followed within 48 hours of preop SRS. Concurrent unresected BM were treated with SRS alone according to standard of care specific to the treating institution. Treatment of subsequent LMD was not standardized and was determined on a case-by-case basis by the treating physicians.
Follow-Up
Patients were generally evaluated with clinical examination and MRI of the brain with and without contrast 6 to 12 weeks after SRS. Patients were then followed with regular assessments and MRI brain imaging every 3–4 months thereafter, unless clinically indicated at an earlier timepoint.
Statistical Analyses
LMD was defined as new, abnormal leptomeningeal enhancement consistent with malignant leptomeningeal involvement ≥5 mm away from the SRS treated prescription isodose line and ≥5 mm away from the surgical corridor for superficial lesions (defined as extending to within 5 mm of the pia mater). This definition is consistent with those used by the committees of Response Assessment in Neuro-Oncology (RANO) and the European Association for Neuro-Oncology (EANO)–European Society for Medical Oncology,27,28 with the exception of requiring ≥5 mm away from the surgical corridor for superficial lesions in order to not have confounding of LMD diagnosis due to local cavity recurrence.
LMD was classified as either classical or nodular based on MRI appearance according to a guide developed by 4 of the study authors for the purposes of standardization (B.E.T., S.H.B., R.S.P., S.G.S., see Supplementary material). Nodular LMD (nLMD) was defined as new focal extra-axial distinct nodular enhancing lesions located on the meninges or ependyma. Classical LMD (cLMD) was akin to “sugarcoating” enhancement and was defined as new linear or curvilinear enhancement of the leptomeninges involving the sulci of the cerebral hemispheres, cranial nerves, brainstem, cerebellar folia, or ependyma.27,28 Extent of surgical resection was based on the neurosurgeon operative note and immediate postoperative cranial imaging report. Gross tumor volume (GTV) was defined as the volume of the target postoperative cavity prior to subsequent margin expansion (if applied) for postoperative SRS and as the volume of the target intact brain metastasis for preoperative SRS.
Time 0 was defined as date of initial LMD recurrence. Proportions between groups were compared using the chi-square or Fisher exact test. OS was estimated using the Kaplan–Meier method, with patients censored at the time they were last known alive. Curves were compared using the log-rank test. Multivariable analysis for OS was performed using the Cox model. Known prognostic factors (age, recursive partitioning analysis [RPA] class,29 primary tumor histology, and number of BM) were included in the model along with the study variables of interest (pattern of LMD failure and type of salvage cranial RT). No variable selection was used as the goal was to determine the significance of the study variables of interest in the setting of known prognostic factors. Type of salvage cranial RT was categorized as WBRT or focal RT. WBRT included any regimen that included WBRT as part of initial salvage (such as WBRT alone or craniospinal RT). Focal RT included SRS or partial brain RT. All analyses were carried out using SPSS version 24 statistical software (IBM). Significance testing was 2-sided, with P-values ≤0.05 considered statistically significant. The manuscript was structured and results were reported according to the guidelines of STROBE (STrengthening the Reporting of OBservational studies in Epidemiology).30
Results
Patient Characteristics
A total of 147 patients from 7 academic institutions were included (Table 1). The vast majority of patients were treated with postoperative SRS (93.9%), with the remainder treated with preoperative SRS (6.1%). The crude incidence of LMD for patients treated with postoperative SRS (n = 138) ranged from 13% to 34% based on institution. The overall crude LMD incidence rate for patients treated with postoperative SRS was 20.8%. Non–small cell lung cancer (NSCLC; 39.5%), breast (23.8%), and melanoma (15.6%) represented the most common tumor types. Most patients (63.3%) had a single initial BM and most resected BM (81.6%) were superficial (defined as ≤5 mm from the pia mater). Median time from postop SRS (or from resection after preop SRS) to LMD was 5.6 months (interquartile range [IQR] 3.2–11). LMD was diagnosed by MRI alone in 83% of patients and by MRI and CSF in 17%. Fifty-seven patients (38.8%) underwent the MRI that demonstrated LMD due to development of symptoms, while 90 (61.2%) received diagnoses on imaging as part of routine surveillance.
Table 1.
Patient, tumor, and treatment characteristics
| Variable | Number or Median | Percent or IQR |
|---|---|---|
| Patients in cohort | 147 | 100% |
| Patients who received LMD salvage therapy | 129 | 87.8% |
| Patients per institution (n = 147) | ||
| Levine Cancer Institute | 6 | 4.1% |
| Stanford | 33 | 22.4% |
| UAB | 32 | 21.8% |
| Emory | 29 | 19.7% |
| Mayo | 11 | 7.5% |
| Beaumont | 29 | 19.7% |
| Cone Health | 7 | 4.8% |
| Sex | ||
| Male | 52 | 35.4% |
| Female | 95 | 64.6% |
| Primary site | ||
| NSCLC | 58 | 39.5% |
| Breast | 35 | 23.8% |
| Melanoma | 23 | 15.6% |
| Renal cell | 4 | 2.7% |
| Gastrointestinal | 17 | 11.6% |
| Other | 10 | 6.8% |
| If NSCLC (n = 58), histology | ||
| Squamous cell | 9 | 15.5% |
| Adenocarcinoma | 44 | 75.9% |
| Large cell NOS | 4 | 6.9% |
| Unknown | 1 | 1.7% |
| Non-squamous NSCLC (n = 44), molecular status | ||
| EGFR mutated | 5 | 11.4% |
| ALK positive | 0 | 0% |
| Unknown status | 30 | 68.2% |
| Breast cancer receptor status (n = 35) | ||
| Estrogen receptor positive | 16 | 45.7% |
| HER-2 positive | 21 | 60% |
| Melanoma molecular status (n = 23) | ||
| BRAF mutated | 9 | 39.1% |
| BRAF unknown | 13 | 56.5% |
| RPA class at time of SRS | ||
| 1 | 47 | 32% |
| 2 | 93 | 63.3% |
| 3 | 3 | 2% |
| Unknown | 4 | 2.7% |
| Extent of index lesion resection | ||
| Gross total | 118 | 80.3% |
| Subtotal | 25 | 17% |
| Unknown | 4 | 2.7% |
| Type of surgery | ||
| Piecemeal | 73 | 49.7% |
| Enbloc | 31 | 21.1% |
| Unknown | 43 | 29.3% |
| Timing of SRS | ||
| Postoperative | 138 | 93.9% |
| Preoperative | 9 | 6.1% |
| LMD incidence (crude) per institution in postoperative SRS subset (n = 138)*# | ||
| Stanford | 33 of 230 | 14.3% |
| UAB | 31 of 90 | 34.4% |
| Emory | 29 of 118 | 24.6% |
| Mayo | 11 of 45 | 24.4% |
| Beaumont | 29 of 141 | 20.6% |
| Cone Health | 5 of 40 | 12.5% |
| Total | 138 of 664 | 20.8% |
| Index brain metastasis location | ||
| Frontal | 38 | 25.9% |
| Parietal | 23 | 15.6% |
| Temporal | 28 | 19% |
| Occipital | 15 | 10.2% |
| Cerebellum | 42 | 28.6% |
| Other | 1 | 0.7% |
| Total number of BM treated in initial SRS session | ||
| 1 | 93 | 63.3% |
| 2 | 29 | 19.7% |
| 3 | 14 | 9.5% |
| 4 | 3 | V |
| ≥5 | 8 | 5.4% |
| Depth of index BM from pial surface | ||
| ≤5 mm | 120 | 81.6% |
| >5 mm | 21 | 14.3% |
| Unknown | 6 | 4.1% |
| LMD treatment type for patients who underwent salvage treatment (n = 129) | ||
| Craniospinal RT | 13 | 10.1% |
| SRS | 35 | 27.1% |
| Spine only RT | 3 | 2.3% |
| WBRT | 60 | 46.5% |
| Surgery | 1 | 0.8% |
| Partial brain RT | 4 | 3.1% |
| Chemotherapy only | 10 | 7.8% |
| Surgery + SRS | 1 | 0.8% |
| WBRT + SRS | 2 | 1.6% |
| LMD treatment type in patients with cranial imaging follow-up (n = 101) | ||
| Craniospinal RT | 10 | 9.9% |
| SRS | 31 | 30.7% |
| Spine only RT | 3 | 3% |
| WBRT | 43 | 42.6% |
| Surgery | 1 | 1% |
| Partial brain RT | 2 | 2% |
| Chemotherapy only | 8 | 7.9% |
| Surgery + SRS | 1 | 1% |
| WBRT + SRS | 2 | 2% |
| Year of LMD diagnosis/treatment | ||
| 2006–2010 | 30 | 20.4% |
| 2011–2014 | 66 | 44.9% |
| 2015–2017 | 51 | 34.7% |
| Craniospinal RT | ||
| Median dose (Gy) | 30 | 30–35 |
| Median fractions | 12 | 10–14 |
| SRS | ||
| Median dose (Gy) | 20 | 18–24 |
| Median fractions | 1 | 1–1 |
| WBRT | ||
| Median dose (Gy) | 30 | 30–35 |
| Median fractions | 10 | 10–15 |
| Age (y) | 58 | 48–67 |
| Interval from initial surgery to SRS (d)* | 27 | 18–37 |
| Index GTV volume (cc) | 11.1 | 6.7–20.7 |
| Interval from initial SRS to initial LMD (mo) | 5.6 | 3.2–11 |
| Interval from initial LMD to second LMD if occurred (n = 50, mo) | 5.5 | 2.8–9.4 |
UAB = University of Alabama at Birmingham, NOS = not otherwise specified, EGFR = epidermal growth factor receptor, ALK = anaplastic lymphoma kinase, HER-2 = human epidermal growth factor receptor 2, GTV = gross tumor volume (defined as the treated cavity for postoperative SRS and the intact brain metastasis for preoperative SRS).
*In patients who received postoperative SRS.
#Levine Cancer Institute contributed only preoperative SRS cases.
Radiographic Presentation of Leptomeningeal Disease
Sixty-three patients (42.9%) presented with cLMD, while 84 (57.1%) presented with nLMD (Fig. 1 and 2). Within the nLMD subset (n = 84), the median number of nodules was 2 (IQR 1–4), the median distance between the surgical corridor and the closest nodule was 2 cm (IQR 1–4.7), with 59 patients (70.2%) having LMD nodules within 5 cm of the surgical corridor. Patient, treatment, and tumor characteristics by pattern of radiographic LMD are available in Supplementary Table 2.
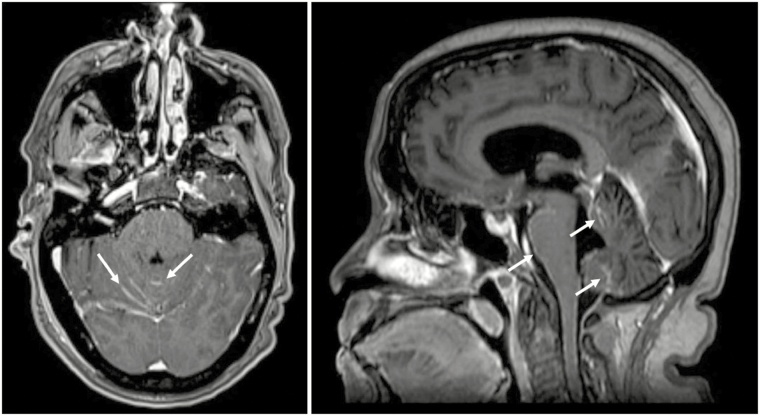
Fig. 1.
MRI T1 post-contrast example of classical (“sugarcoating”) pattern of leptomeningeal disease (cLMD). (A) Axial orientation. (B) Sagittal orientation. White arrows point to areas of abnormal linear cerebellar folia and brainstem enhancement consistent with cLMD.
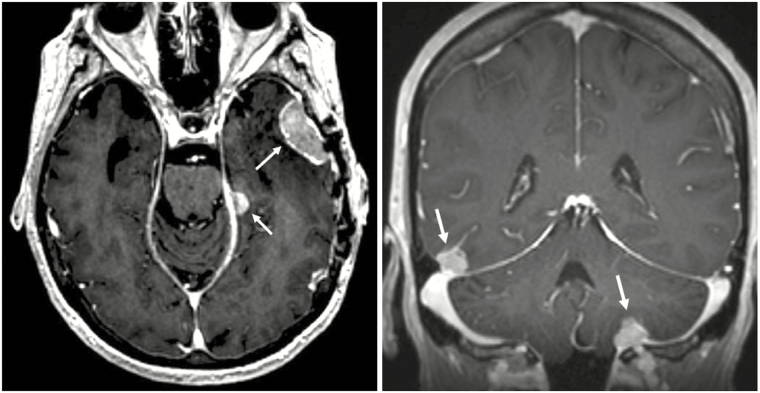
Fig. 2.
MRI T1 post-contrast example of nodular pattern of leptomeningeal disease (nLMD). (A) Axial orientation. (B) Coronal orientation. White arrows point to areas of extra-axial meningeal based nodular enhancement consistent with nLMD.
Clinical Presentation of Leptomeningeal Disease
The majority of patients (60%) were symptomatic at the time of LMD diagnosis (Table 2). Of the 88 symptomatic patients, headache was the most common symptom (56%), followed by cranial nerve deficit (28.4%), balance/ataxia issues (27.3%), and nausea (13.6%). Patients with cLMD were significantly more likely to be symptomatic compared with nLMD (71.4% vs 51.2%, P = 0.01). The pattern of specific symptoms did not differ between LMD types except for nausea, which was significantly more common with cLMD (26.7% vs 2.3%, P = 0.002; Supplementary Table 3).
Table 2.
Symptom presentation at time of initial and second LMD recurrence
| Number | Percent | |
|---|---|---|
| Patients symptomatic at time of initial LMD | 88 | 59.9 |
| Symptoms at initial LMD (percentage of 88 symptomatic patients)* | ||
| Headache | 51 | 58 |
| Cranial nerve deficit | 25 | 28.4 |
| Nausea | 13 | 14.8 |
| Ataxia/balance | 26 | 29.5 |
| Hydrocephalus | 3 | 3.4 |
| Other | 40 | 45.5 |
| Patients symptomatic at time of second LMD (of 50 patients with second LMD) | 34 | 68 |
| Symptoms at second LMD (percentage of 34 symptomatic patients)* | ||
| Headache | 11 | 32.4 |
| Cranial nerve deficit | 8 | 23.5 |
| Nausea | 6 | 17.6 |
| Ataxia/balance | 10 | 29.4 |
| Hydrocephalus | 2 | 5.9 |
| Other | 16 | 47.1 |
*Percents may add up to more than 100% due to multiple symptoms in a single patient.
Treatment of Initial Leptomeningeal Disease
Of the 147 patients, 129 (87.8%) received salvage therapy for LMD (Table 1). More patients with cLMD received no salvage therapy compared with nLMD (20.6% vs 6%, P = 0.01). The most common treatments were WBRT alone (46.5%), SRS (27.1%), and craniospinal RT (10.1%). Of the 129 patients who received LMD salvage therapy, 75 (58.1%) received a regimen that included WBRT, while 40 (31%) received focal cranial RT (SRS or partial brain RT). Univariate analysis of factors for type of cranial RT salvage showed significant associations only for presence or absence of LMD-associated symptoms (P = 0.001) and radiographic pattern of LMD (P < 0.001). Patients who received WBRT were significantly more likely to be symptomatic at presentation and/or have cLMD. For patients with nLMD who received cranial RT based salvage (n = 73), 35 (47.9%) received WBRT while 38 (52.1%) received focal RT. Comparatively, of the 42 patients with cLMD who received cranial RT based salvage, 40 (95.2%) received WBRT while 2 (4.8%) received focal RT.
LMD Recurrence Based on Initial Pattern and Salvage Type
Of the cohort of 147 patients, 101 (67%) had a known pattern of LMD recurrence, received salvage therapy, and had follow-up cranial imaging. The median cranial imaging follow-up period after initial LMD for these patients was 5.8 months (IQR 2.3–11.7). For patients with nLMD treated with salvage RT (n = 61), there was a trend toward significance for rate of second LMD recurrence based on initial salvage therapy. Twelve of 28 patients (42.9%) treated with WBRT had second LMD recurrence compared with 22 of 33 patients (66.7%) treated with focal RT (P = 0.08). About half (58.3%) of second LMD recurrences after WBRT were again nodular compared with 86.4% after focal RT (P = 0.1). Of the 27 patients with cLMD treated with WBRT, 10 (37%) had second LMD recurrence, of which 9 (90%) were again cLMD. There was only 1 evaluable patient with cLMD treated with focal RT who recurred with second cLMD. Twenty-two of 55 patients (40%) treated with WBRT with any pattern of initial LMD experienced second LMD recurrence compared with 23 of 34 patients (67.6%) treated with focal RT (P = 0.02). For the 50 patients who experienced second LMD, the median time from initial to second LMD was 5.5 months (IQR 2.8–9.4). The majority of patients (68%) were symptomatic at time of second LMD (Table 2).
Survival Outcomes Based on Initial LMD Pattern and Salvage Type
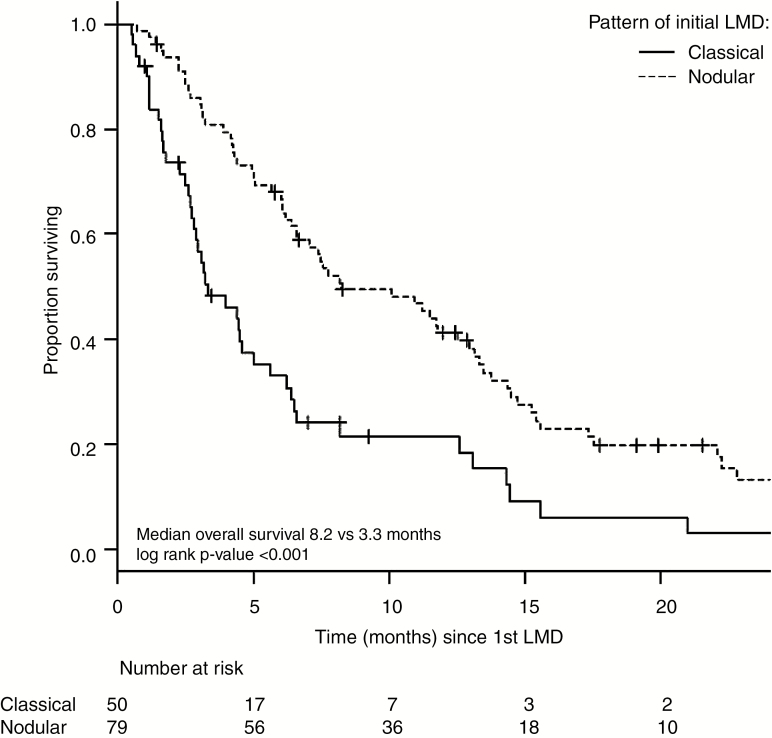
The median OS for the entire cohort of 147 patients was 5.4 months (95% confidence interval [CI]: 4.1–6.8). Patients who received salvage treatment had significantly longer median OS compared with those who did not (6.4 vs 0.9 mo, P < 0.001). Of the 129 patients who received salvage treatment, patients with nLMD had significantly longer survival than those with cLMD (median OS 8.2 vs 3.3 mo, P < 0.001; Fig. 3). Of the 115 patients who received cranial RT based salvage, patients treated with focal RT had significantly longer OS compared with those treated with WBRT (median OS 12.5 mo vs 4.4 mo, P < 0.001). Within the subset of nLMD treated with RT salvage (n = 73), OS was also significantly longer for focal RT compared with WBRT (median OS 13 vs 6.6 mo, P = 0.01). On multivariable analysis for OS in 115 patients who underwent salvage RT controlling for known prognostic factors (age, RPA class, number of BM, and primary tumor histology), pattern of initial LMD (nodular vs classical) was a significant predictor of OS (hazard ratio [HR] 0.59, 95% CI: 0.34–0.98, P = 0.04; Table 3).
Fig. 3.
Overall survival based on radiographic pattern of initial leptomeningeal disease (LMD) in patients who received salvage therapy (n = 129). Curves truncated at 24 months.
Table 3.
Multivariable analysis for OS in patients who underwent salvage radiotherapy (n = 115)
| Variable | Hazard Ratio | 95% CI | P-value |
|---|---|---|---|
| Age (continuous) | 1.005 | 0.98–1.03 | 0.63 |
| RPA class | |||
| Class 1 | Reference | 0.63 | |
| Class 2 | 0.88 | 0.56–1.39 | 0.59 |
| Class 3 | 1.53 | 0.4–5.91 | 0.54 |
| Pattern of initial LMD | |||
| Nodular (vs classical) | 0.59 | 0.34–0.98 | 0.04 |
| Primary tumor type | |||
| NSCLC | Reference | 0.002 | |
| Breast | 0.69 | 0.36–1.32 | 0.26 |
| Melanoma | 2.4 | 1.12–5.17 | 0.03 |
| Gastrointestinal | 1.47 | 0.75–2.91 | 0.26 |
| Other | 0.42 | 0.18–1.01 | 0.054 |
| Type of RT salvage | |||
| Focal RT (vs WBRT) | 0.65 | 0.4–1.06 | 0.09 |
| Number of BM at initial SRS | |||
| 1 | Reference | 0.91 | |
| 2–4 | 0.91 | 0.55–1.5 | 0.71 |
| ≥5 | 1.07 | 0.41–2.78 | 0.89 |
Discussion
The development of leptomeningeal disease from solid cancer is generally viewed as a late stage event with median OS with treatment of approximately 3–4 months.31 The expected median OS for untreated patients with LMD is approximately 4–6 weeks.32 The vast majority of data comes from cases where the LMD developed through classical means, that is, via hematogenous spread or direct extension of parenchymal brain metastases to the subarachnoid space. Surgical resection of BM has been demonstrated as an independent risk factor for LMD development relative to nonsurgical management (eg, SRS) in multiple studies.14,15,18 These findings suggest that surgery may spill tumor cells from the BM into the CSF at the time of resection, leading to increased risk of LMD. This pattern of recurrence was not as relevant when adjuvant WBRT was the standard of care due to radiating the entire intracranial CSF space, but has become more apparent with increased use of postoperative SRS.21 Surgery-associated LMD is a mechanism of LMD development that is distinct from the classical pathogenesis via hematogenous spread or direct tumor extension.
The risk of LMD after surgery and SRS for BM ranges from approximately 5% to up to 31%.9,19,25 Aside from surgical resection itself, the most consistent risk factor for LMD across studies appears to be breast cancer histology.12,16–20 Retrospective and prospective studies reporting LMD in this setting are limited by the relatively low absolute number of patients who develop LMD, generally in the range of 20–40 patients per study from high volume institutions.9,13,16,17,19 We combined patient data from multiple high volume institutions in order to have a large enough patient population to perform comparative analyses and make meaningful conclusions. The focus of this study was specifically on the cohort of patients who developed LMD. We intentionally did not include patients who did not develop LMD, as most of the included institutions have separately reported on these patients in previous publications (Supplementary Table 1). The influence of proposed risk factors for LMD (histology, location, surgery type, etc) on the current patient cohort will be the subject of future studies.
A primary finding of this study is that the radiographic diagnosis of LMD is clinically relevant in that 60% of patients were symptomatic at the time of LMD diagnosis. Approximately 40% of the MRI that diagnosed LMD was performed for symptoms rather than surveillance. The presenting constellation of symptoms (headache, cranial nerve deficit, nausea, ataxia/balance) was consistent with other published studies of LMD presentation.31 In addition, the median OS of patients who did not receive salvage therapy for LMD was only about 4 weeks.
Another novel finding of this study was the demonstration of 2 distinct radiographic patterns of LMD recurrence not previously well described. Classical LMD is more consistent with typical “sugarcoating” LMD, while nLMD seems to be a pattern more associated with postsurgical LMD development. In addition to the radiographic differences, these 2 patterns seem to have distinct biologic behavior in that patients with nLMD were less likely to be symptomatic and had survival outcomes that were significantly better compared with cLMD (median OS 8.2 vs 3.3 mo, P < 0.001). The median OS for patients with cLMD who received treatment was on par with the 3- to 4-month median OS expectation reported in the literature for patients with LMD across a number of studies.31,32 However, those with nLMD had median OS much longer than expected and on par with patients who had non-LMD parenchymal BM.3–6 We hypothesize that radiographic cLMD represents a tumor aggressiveness and phenotype similar to classic secondary LMD with similar outcome expectations. In contrast, those with nLMD may have experienced tumor spillage into the CSF at the time of surgery and this radiographic LMD is a distinct manifestation of that process with better outcome expectations in comparison to a late stage LMD event from hematogenous spread or direct tumor extension into the subarachnoid space.
There is no single standard of care for LMD, but WBRT and/or involved site RT to bulky or symptomatic spinal sites is a mainstay of initial therapy.33 However, it is now well established that WBRT is associated with neurocognitive detriment compared with non-WBRT local therapies as early as 3–4 months posttreatment.4,5,11 The vast majority of patients in this study with cLMD did undergo WBRT, but the proportions were more balanced for treatment with focal RT and WBRT for patients with nLMD. Patients treated with focal RT (mostly SRS) were more likely to have second LMD recurrence in general, and more specifically nLMD recurrence, compared with WBRT. However, there was no OS detriment associated with the use of focal RT for nLMD compared with WBRT-containing regimens. These findings are similar to the findings from multiple trials of local therapy versus local therapy and WBRT for BM where the intracranial recurrence rates were higher without WBRT, but there was no associated OS detriment with the omission of WBRT.3–6,8,11 Patients with nLMD who have a median OS of over 8 months after the initial LMD event are at risk of the intermediate and long-term potential side effects of WBRT, which should be considered when recommending treatment.
The strengths of this study include its large patient population, multi-institutional design, consistent cranial imaging follow-up regimen, and uniform protocol definition of LMD and radiographic LMD subtypes. The radiographic definition of LMD, especially in the postsurgical setting, is not standardized, and recent RANO and EANO publications used generalized definitions consistent with the one used in this study.27,28 In addition, although only 17% of patients had CSF cytology confirmation of LMD, radiographic findings in the appropriate clinical and symptom context is sufficient to diagnose LMD according to consensus recommendations and real-world clinical reports.28,34 The limitations of this study include those inherent to retrospective studies, including selection bias for LMD treatment choice, potential confounding for OS endpoints due to the nonrandomized nature of the study, lack of quality of life and neurocognitive data to compare for the various groups, lack of detailed molecular status for several tumor histologies, and lack of complete data for some risk factors for LMD, such as type of surgical resection and hemorrhagic/cystic features.
Conclusions
LMD failure after surgery and SRS for brain metastases is an increasingly recognized event. Most patients are symptomatic at the time of LMD diagnosis. Nodular LMD is a distinct pattern of LMD associated with surgery and postoperative SRS that is less likely to be symptomatic and has better OS outcomes than classical “sugarcoating” LMD, regardless of initial RT salvage treatment. Focal RT, used primarily for nLMD, was associated with increased second LMD recurrence compared with WBRT. However, there was no OS detriment associated with the use of focal RT for nLMD compared with WBRT-containing regimens. Focal cranial RT (SRS or partial brain) for nLMD recurrence after surgery and SRS for BM may be a reasonable alternative to WBRT.
Funding
No specific funding was used for the conduct of this study.
Conflict of interest statement. No conflict of interest exists for any of the listed authors.
Authorship statement: Study design: RSP, ALA, SGS, SHB Acquisition of data: all authors Statistical analyses: RSP Interpretation of the data: RSP, ALA, SGS, SHB Drafting of the initial manuscript: RSP Review and revision of the manuscript: all authors Approval of the final manuscript: all authors
Supplementary Material
Partially presented in abstract form at the American Society of Clinical Oncology (ASCO) Annual Meeting 2018 and at the American Society of Therapeutic Radiation Oncology (ASTRO) Annual Meeting 2018.
References
- 1. Nayak L, Lee EQ, Wen PY. Epidemiology of brain metastases. Curr Oncol Rep. 2012;14(1):48–54. [DOI] [PubMed] [Google Scholar]
- 2. Smedby KE, Brandt L, Backlund ML, Blomqvist P. Brain metastases admissions in Sweden between 1987 and 2006. Br J Cancer. 2009;101(11):1919–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aoyama H, Shirato H, Tago M, et al. . Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295(21):2483–2491. [DOI] [PubMed] [Google Scholar]
- 4. Brown PD, Jaeckle K, Ballman KV, et al. . Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: a randomized clinical trial. JAMA. 2016;316(4):401–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chang EL, Wefel JS, Hess KR, et al. . Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10(11):1037–1044. [DOI] [PubMed] [Google Scholar]
- 6. Kocher M, Soffietti R, Abacioglu U, et al. . Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol. 2011;29(2):134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Soffietti R, Kocher M, Abacioglu UM, et al. . A European Organisation for Research and Treatment of Cancer phase III trial of adjuvant whole-brain radiotherapy versus observation in patients with one to three brain metastases from solid tumors after surgical resection or radiosurgery: quality-of-life results. J Clin Oncol. 2013;31(1):65–72. [DOI] [PubMed] [Google Scholar]
- 8. Kayama T, Sato S, Sakurada K, et al. . Effects of surgery with salvage stereotactic radiosurgery versus surgery with whole-brain radiation therapy in patients with one to four brain metastases (JCOG0504): a phase III, noninferiority, randomized controlled trial. J Clin Oncol. 2018:JCO2018786186. [DOI] [PubMed] [Google Scholar]
- 9. Mahajan A, Ahmed S, McAleer MF, et al. . Post-operative stereotactic radiosurgery versus observation for completely resected brain metastases: a single-centre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017;18(8):1040–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Patchell RA, Tibbs PA, Regine WF, et al. . Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA. 1998;280(17):1485–1489. [DOI] [PubMed] [Google Scholar]
- 11. Brown PD, Ballman KV, Cerhan JH, et al. . Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC·3): a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017;18(8):1049–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Foreman PM, Jackson BE, Singh KP, et al. . Postoperative radiosurgery for the treatment of metastatic brain tumor: evaluation of local failure and leptomeningeal disease. J Clin Neurosci. 2018;49:48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Johnson MD, Avkshtol V, Baschnagel AM, et al. . Surgical resection of brain metastases and the risk of leptomeningeal recurrence in patients treated with stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2016;94(3):537–543. [DOI] [PubMed] [Google Scholar]
- 14. Huang AJ, Huang KE, Page BR, et al. . Risk factors for leptomeningeal carcinomatosis in patients with brain metastases who have previously undergone stereotactic radiosurgery. J Neurooncol. 2014;120(1):163–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ma R, Levy M, Gui B, et al. . Risk of leptomeningeal carcinomatosis in patients with brain metastases treated with stereotactic radiosurgery. J Neurooncol. 2018;136(2):395–401. [DOI] [PubMed] [Google Scholar]
- 16. Ojerholm E, Lee JY, Thawani JP, et al. . Stereotactic radiosurgery to the resection bed for intracranial metastases and risk of leptomeningeal carcinomatosis. J Neurosurg. 2014;121(Suppl):75–83. [DOI] [PubMed] [Google Scholar]
- 17. Patel KR, Burri SH, Asher AL, et al. . Comparing preoperative with postoperative stereotactic radiosurgery for resectable brain metastases: a multi-institutional analysis. Neurosurgery. 2016;79(2):279–285. [DOI] [PubMed] [Google Scholar]
- 18. Suki D, Hatiboglu MA, Patel AJ, et al. . Comparative risk of leptomeningeal dissemination of cancer after surgery or stereotactic radiosurgery for a single supratentorial solid tumor metastasis. Neurosurgery. 2009; 64(4):664–674; discussion 674-666. [DOI] [PubMed] [Google Scholar]
- 19. Atalar B, Modlin LA, Choi CY, et al. . Risk of leptomeningeal disease in patients treated with stereotactic radiosurgery targeting the postoperative resection cavity for brain metastases. Int J Radiat Oncol Biol Phys. 2013;87(4):713–718. [DOI] [PubMed] [Google Scholar]
- 20. Press RH, Zhang C, Chowdhary M, et al. . Hemorrhagic and cystic brain metastases are associated with an increased risk of leptomeningeal dissemination after surgical resection and adjuvant stereotactic radiosurgery. Neurosurgery. 2018. doi: 10.1093/neuros/nyy436. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 21. Patel KR, Prabhu RS, Kandula S, et al. . Intracranial control and radiographic changes with adjuvant radiation therapy for resected brain metastases: whole brain radiotherapy versus stereotactic radiosurgery alone. J Neurooncol. 2014;120(3):657–663. [DOI] [PubMed] [Google Scholar]
- 22. Turner BE, Prabhu RS, Burri SH, et al. . Nodular leptomeningeal disease – a distinct pattern of recurrence after post-resection stereotactic radiosurgery for brain metastases: a multi-institutional study of inter-observer reliability. Int J Radiat Oncol Biol Phys. 2018;102(3):e363–e364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Asher AL, Burri SH, Wiggins WF, et al. . A new treatment paradigm: neoadjuvant radiosurgery before surgical resection of brain metastases with analysis of local tumor recurrence. Int J Radiat Oncol Biol Phys. 2014;88(4):899–906. [DOI] [PubMed] [Google Scholar]
- 24. Patel KR, Burri SH, Boselli D, et al. . Comparing pre-operative stereotactic radiosurgery (SRS) to post-operative whole brain radiation therapy (WBRT) for resectable brain metastases: a multi-institutional analysis. J Neurooncol. 2017;131(3):611–618. [DOI] [PubMed] [Google Scholar]
- 25. Prabhu RS, Patel KR, Press RH, et al. . Preoperative vs postoperative radiosurgery for resected brain metastases: a review. Neurosurgery. 2019;84(1):19–29. [DOI] [PubMed] [Google Scholar]
- 26. Prabhu RS, Miller KR, Asher AL, et al. . Preoperative stereotactic radiosurgery before planned resection of brain metastases: updated analysis of efficacy and toxicity of a novel treatment paradigm. J Neurosurg. 2018:1–8. doi: 10.3171/2018.7.JNS181293. [DOI] [PubMed] [Google Scholar]
- 27. Chamberlain M, Junck L, Brandsma D, et al. . Leptomeningeal metastases: a RANO proposal for response criteria. Neuro Oncol. 2017;19(4):484–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Le Rhun E, Weller M, Brandsma D, et al. . ANO-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up of patients with leptomeningeal metastasis from solid tumours. Ann Oncol. 2017;28(suppl_4):iv84–iv99. [DOI] [PubMed] [Google Scholar]
- 29. Gaspar L, Scott C, Rotman M, et al. . Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37(4):745–751. [DOI] [PubMed] [Google Scholar]
- 30. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–1457. [DOI] [PubMed] [Google Scholar]
- 31. Wang N, Bertalan MS, Brastianos PK. Leptomeningeal metastasis from systemic cancer: review and update on management. Cancer. 2018;124(1):21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gleissner B, Chamberlain MC. Neoplastic meningitis. Lancet Neurol. 2006;5(5):443–452. [DOI] [PubMed] [Google Scholar]
- 33. National Comprehensive Cancer Network (NCCN). Central Nervous System Cancers Version 1.2018 2018; https://www.nccn.org/professionals/physician_gls/pdf/cns.pdf. Accessed September 20, 2018.
- 34. Hyun JW, Jeong IH, Joung A, Cho HJ, Kim SH, Kim HJ. Leptomeningeal metastasis: clinical experience of 519 cases. Eur J Cancer. 2016;56:107–114. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.