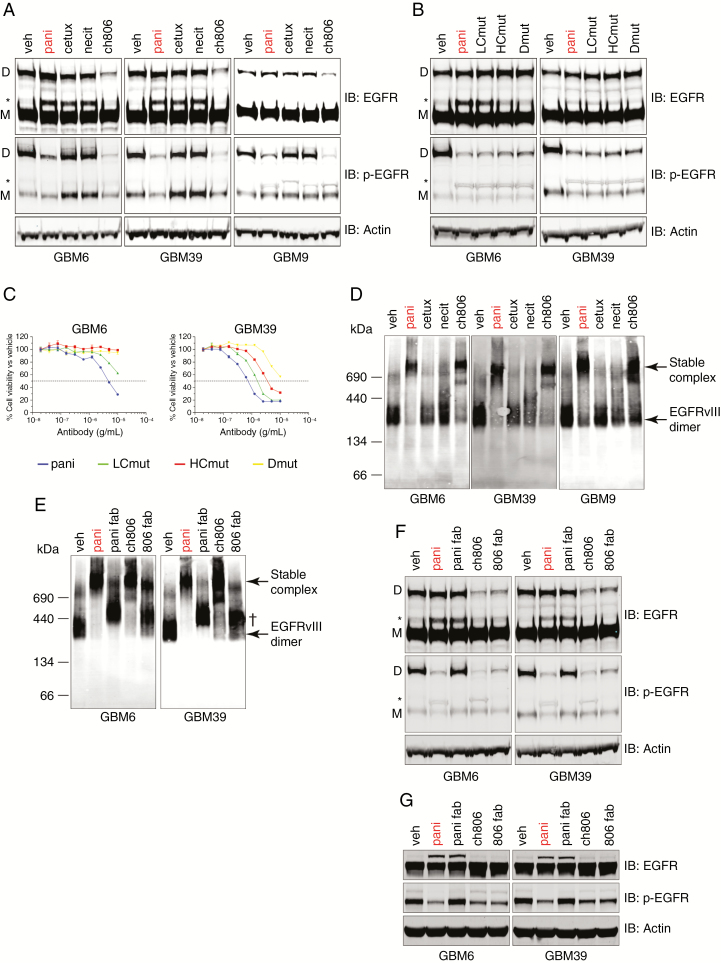
Fig. 4.
Interrogation of the mechanism of action of how panitumumab and ch806 are able to neutralize EGFRvIII. (A) Non-reducing SDS-PAGE and western analyses of total and pY1068 wtEGFR and EGFRvIII in 3 EGFRvIII-expressing gliomasphere cell lines following treatment. (B) Non-reducing SDS-PAGE and western analyses of total and pY1068 wtEGFR and EGFRvIII in 3 EGFRvIII-expressing gliomasphere cell lines following treatment with panitumumab or its mutants. (C) Representative plots for the in vitro inhibition of proliferation in gliomaspheres expressing EGFRvIII after 7 days of treatment with increasing concentrations of panitumumab or its mutants as measured by Vialight assay. Data are presented as percentage of cell viability vs vehicle controls ± s.e.m. Horizontal dashed line = half-maximal inhibitory concentration demarcation. (D) BN-PAGE and native western analyses of total EGFR status in 3 EGFRvIII-expressing gliomasphere cell lines following treatment. Molecular weights in kDa are indicated to the left of panel. (E) BN-PAGE and native western analyses of total EGFR status in 3 EGFRvIII-expressing gliomasphere cell lines following treatment. Molecular weights in kDa are indicated to the left of panel. † = Fab•EGFRvIII complex. (F) Non-reducing SDS-PAGE and western analyses of total and pY1068 wtEGFR and EGFRvIII from the same tests as (E). (G) Reducing SDS-PAGE and western analyses of total and pY1068 wtEGFR and EGFRvIII from the same tests as (E). All treatments for western analyses were conducted for 24 h with 10 µg/mL antibody or molar equivalent Fab (6.6 µg/mL) and actin loading controls included. D = EGFRvIII disulphide-bonded dimer, M = EGFRvIII monomer, * = wtEGFR.