Fig. 1 -.
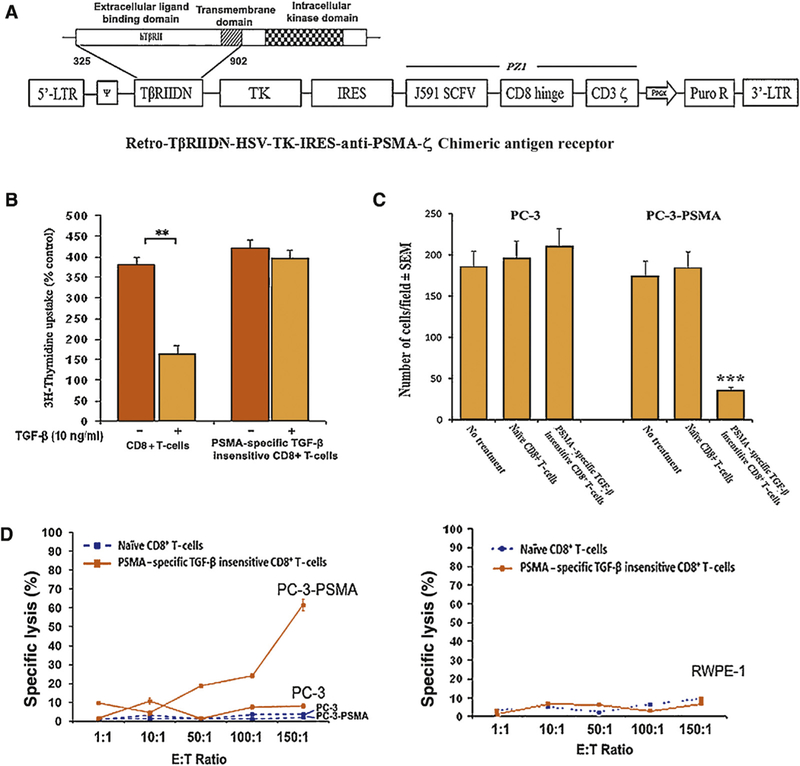
(A) Schematic diagram of the chimeric antigen T-cell receptor retroviral construct dominant negative transforming growth factor-ß type II receptor (TβRIIDN)-thymidine kinase (TK)-internal ribosomal entry sequence (IRES)-antiprostate-specific membrane antigen (PSMA) IgTCR(ζ; PZ1) retroviral construct. A truncated sequence of the human TpRIIDN, lacking the intracellular kinase signaling domain, and HSV-TK1 gene was cloned into the pMig-IRES vector following by PZ1 which is a PSMA-specific fusion receptor that encompasses an scFv derived from the J591 hybridoma, joining the VH and VL fragments through a serine/glycine linker and human CD8α hinge, and the transmembrane domain which links the scFv to the intracellular domain of human TCR CD3ζ thus mediating activation signals upon binding and results in major histocompatibility complex independent activation. Furthermore, we included the gene for TβRIIDN which can bind with TGF-β but will not transmit the signal into the cell, which reduces the sensitivity to TGF-ß, and for safety the herpes simplex virus-1 TK gene. (B) [3H]-Thymidine incorporation into naïve CD8+ T-cells or PSMA-specific TGF-β insensitive CD8+ T-cells before and after the treatment of with TGF-β1. Bars, standard deviation. PSMA-specific TGF-β insensitive CD8+ T-cells are insensitive to the treatment of TGF-β when naïve CD8+ T-cells were inhibited by 57.1%. (C) PC-3 and PC-3-PSMA possessed equal invasive capabilities. There were no significant changes in cell motility through a Matrigel-coated polycarbonate membrane when cocultured with naïve CD8+ T-cells. The invasion of PC-3-PSMA cells, but not PC-3 cells, could be inhibited by coculture with PSMA-specific TGF-β insensitive CD8+ T-cells. Corresponding numbers of invasive cells are listed. (D) In-vitro cytotoxic T-lymphocyte assay. Cytotoxic T-lymphocyte assay was done using the conventional 51Cr release assay (Supplementary data). Naïve CD8+ T-cells and PSMA-specific TGF-β insensitive CD8+ T-cells were cocultured with 51Cr-labeled targets at the specific E/T ratios. PC-3 and PC-3-PSMA cells were used as the targets in the top part of the figure and RWPE-1 cells were used as the targets in the bottom part of the figure. Point, average observations obtained from eight wells; bars, standard deviation. PSMA-specific TGF-p insensitive CD8+ T-cells generated 61.5% specific lysis against PC-3-PSMA but not PC-3 or RWEP-1 when there was lower than 10% lysis found by treating with naïve CD8+ T-cells.
