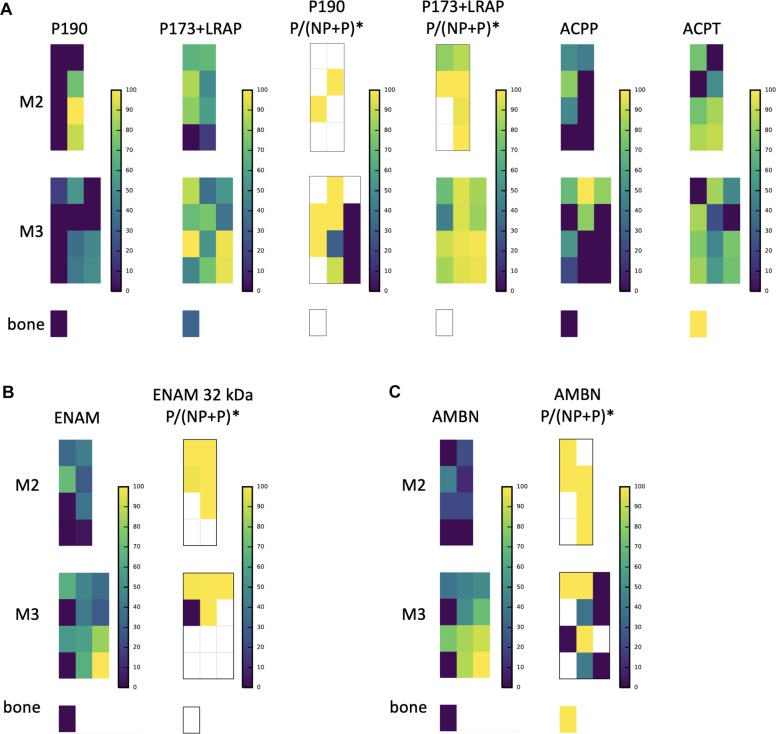
FIGURE 4.
A subsample of protein abundance and posttranslational modification maps in forming teeth. (A) P190 and P173+LRAP abundance distributions are shown on the left, followed by the ratio of phosphorylated form to phosphorylated plus non-phosphorylated (P:P+NP) peptides, and on the right abundance distributions of testicular acid phosphatase precursor (ACPP) and protein (ACPT), which have dephosphorylating functions and are associated with enamel defects when mutated. (B) ENAM distribution shown next to the distribution of the ratio of the phosphorylated to phosphorylated plus non-phosphorylated (P:P+NP) ENAM peptide corresponding to the 32 kDa cleavage product. Our results show that the peptide is present primarily in more mature enamel, consistent with its resistance to MMP20 proteolytic processing, and that it is almost wholly phosphorylated (one unphosphorylated location corresponds to very low abundance overall). (C) AMBN overall abundance followed by the ratio of phosphorylated AMBN. *The ratio of the phosphorylated peptide to total peptide abundance normalized over the entire enamel area analyzed; white space indicates no peptide data at this location.