FIGURE 3.
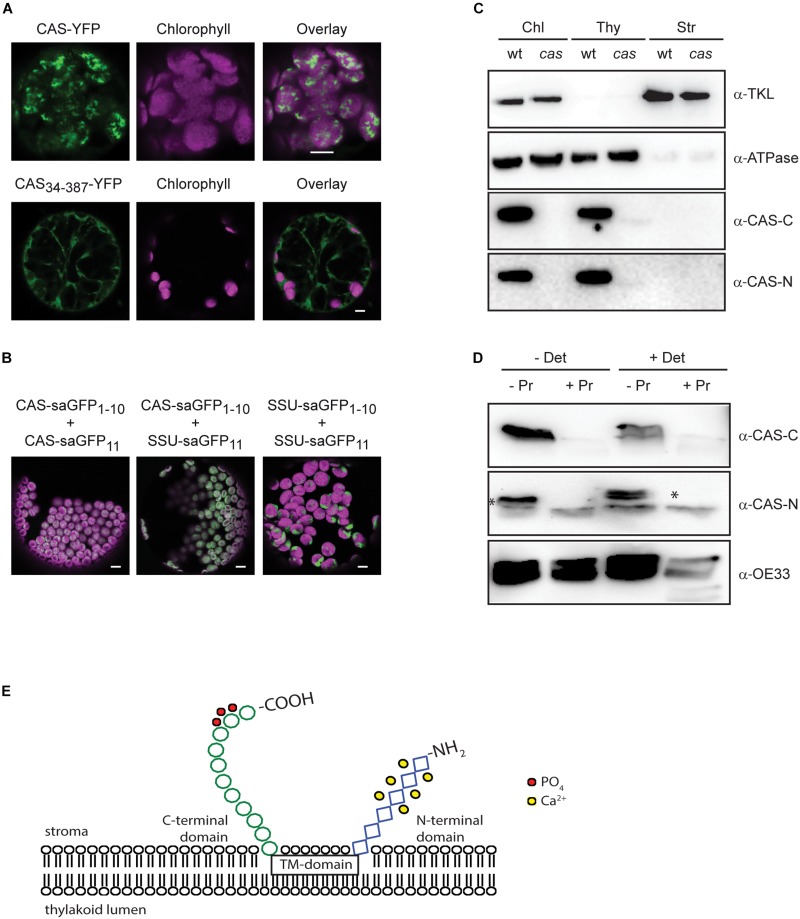
Topology analysis of CAS. (A) Expression of CAS-YFP in tobacco protoplasts shows a clear overlap of the YFP and chlorophyll fluorescence signal confirming the correct targeting of CAS-YFP into the chloroplast. Removal of the predicted transit peptide (CAS34–387-YFP) resulted in a cytosolic localization (white bars indicate 5 μm). (B) Fluorescence analyses of tobacco leaf cell protoplasts co-transformed with the self-assembly GFP pairs CAS-saGFP1–10/CAS-saGFP11, CAS-saGFP1–10/RUBISCO-saGFP11, and RUBISCO-saGFP1–10/Rubisco-saGFP11 confirm that the C-terminus of CAS is exposed to the stromal side of the thylakoid membrane (white bars indicate 5 μm). (C) Isolated chloroplasts from wild type (wt) and cas mutant plants were separated into thylakoid membranes and stroma and all fractions were probed with antibodies directed against the two CAS domains (α-CAS-C; α-CAS-N), beta-subunit of the chloroplast ATP-synthase (α-ATPase) and transketolase (α-TKL). A corresponding Coomassie-stained gel is shown in Supplementary Figure S3. (D) Isolated thylakoid membranes from wild type plants were treated with the protease thermolysin (+/− Pr) in the absence or presence of detergent (+/− Det). All fractions were probed with antibodies against the C- and N-terminal domain of CAS (α-CAS-C, α-CAS-N) as depicted in Figure 1A. The asterisk indicates the specific reaction of the α-CAS-N antibody with CAS. An antibody against the oxygen-evolving system protein 33 (α-OE33) was used as a control to assess the integrity of the thylakoid membrane during the treatment. (E) Topology model of CAS showing the exposure of conserved phosphorylation sites (red circles) onto the stromal surface of the thylakoids. Ca2+-binding to the stromal exposed N-terminal domain is indicated by yellow circles.