FIGURE 7.
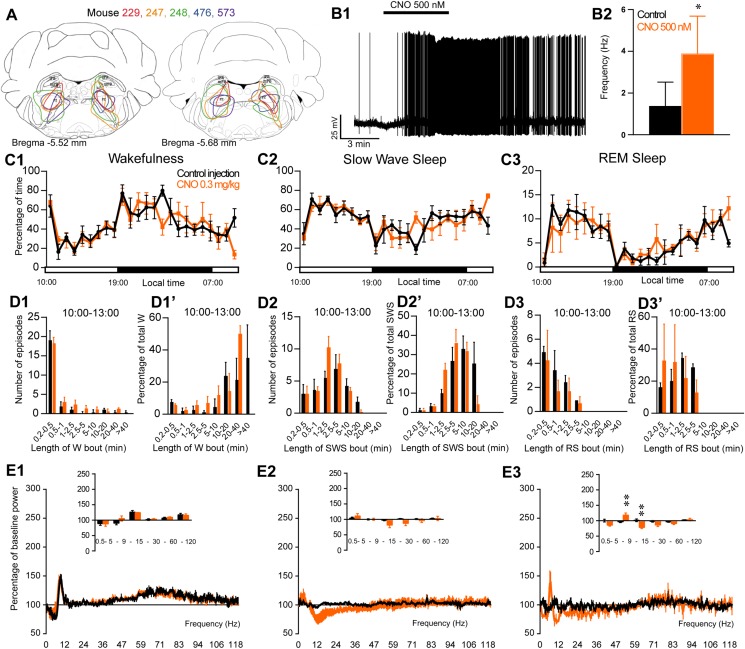
Activation of PZ Phox2B-expressing neurons during the inactive period (10:00 or ZT3). (A) Extent of transduced neurons (mCherry-positive somas) is shown for individual Phox2B-IRES-cre mice that received bilateral injections of hM3Dq-mCherry-AAV into the PZ (PZPhox2B–hM3Dq). (B1) PZPhox2B–hM3Dq whole-cell recording showing an increase in firing frequency in response to bath application of CNO (0.5 μM). (B2) Average firing frequency (±S.E.M.) during the last 2 min of CNO (0.5 μM) application as compared with the 2 min period preceding CNO application (control; N = 5 PZPhox2B–hM3Dq neurons). *p < 0.05 Paired Student’s t-tests. (C) Hourly amount of wakefulness (C1), SWS (C2) and REM sleep (C3) following CNO (0.3 mg/kg, 10 A.M., N = 4 mice) as compared with control injection. (D1–D3) Number of episodes (±S.E.M.) of wakefulness (W), SWS, or REM sleep (RS) in each bout length and (D1’–D3’) time-weighted frequency histograms showing the proportion (±S.E.M.) of W, SWS or RS amounts in each bout length as a percentage of the total amount of W, SWS or RS during the 3 h post-injection period (10:00–19:00; N = 4). (E1–E3) Sleep-wake power spectrum changes over baseline during the 3 h (10:00–13:00) post CNO (0.3 mg/kg, N = 4 mice) injection as compared with control injection; and the quantitative changes (±S.E.M.) in power for the δ (0.4–5 Hz), θ (5–9 Hz), α (9–15 Hz), β (15–30 Hz), low γ (30–60 Hz) and high γ (60–120 Hz) frequency bands (±S.E.M.) following vehicle or CNO (0.3 mg/kg, N = 4 mice) administrations. (C–E) Control injection in Black, CNO injection in orange; ∗∗p < 0.01, two-way ANOVA followed by a post hoc Bonferroni test.