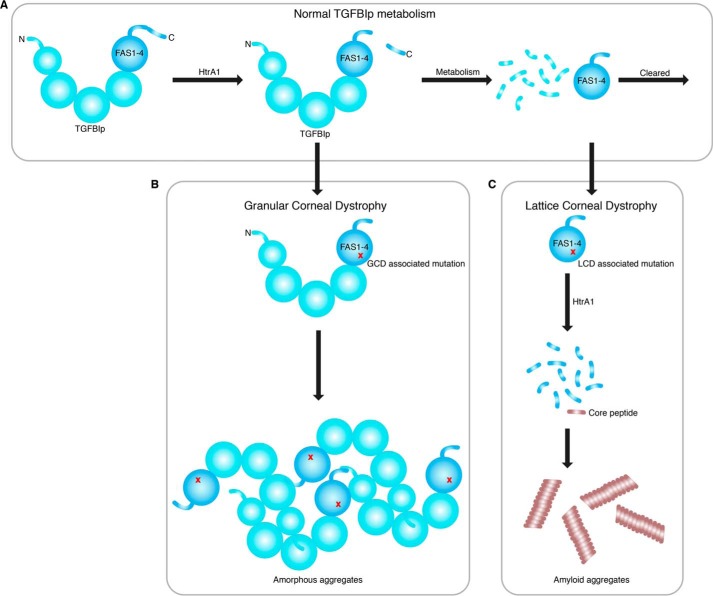
Figure 8.
Proposed mechanisms involved in TGFBI-linked corneal dystrophies. A, normal corneal TGFBIp turnover where TGFBIp is first cleaved in its C-terminal, possibly by HtrA1. Then, it undergoes extensive N-terminal processing by the proteolytic machinery in the cornea, leading to FAS1-4–containing degradation products, which are cleared by an unknown mechanism. B, in GCD, the structural and proteolytic stability of TGFBIp increases, resulting in a slower turnover of the protein. This slower turnover leads to a buildup of TGFBIp, which eventually forms amorphous aggregates upon reaching a critical concentration. C, in the case of LCD, the TGFBIp structure is compromised by a mutation that increases its turnover or leads to alternative degradation pathways. LCD caused by FAS1-4 mutations allows the serine protease HtrA1 to turn over this domain, thereby liberating the amyloidogenic core region of TGFBIp. Upon reaching a critical concentration, the TGFBIp core peptide starts to form amyloid aggregates.