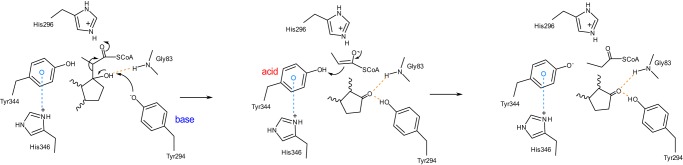
Figure 5.
Proposed retro-aldol cleavage mechanism of Ltp2–ChsH2DUF35. This prediction was made based on the modeling of the 17-HOPC-CoA into the active site and the results obtained from site-directed mutagenesis studies. Tyr-294 deprotonates the C17 hydroxyl group of 17-HOPC-CoA leading to C–C bond cleavage. The second tyrosine residue, Tyr-344, acts as an acid, donating a proton to the enolate anion of the CoA thioester, forming propionyl-CoA and androst-4-ene-3,17-dione. Either Tyr-294 or Tyr-344 could be activated by the nearby histidine residues.