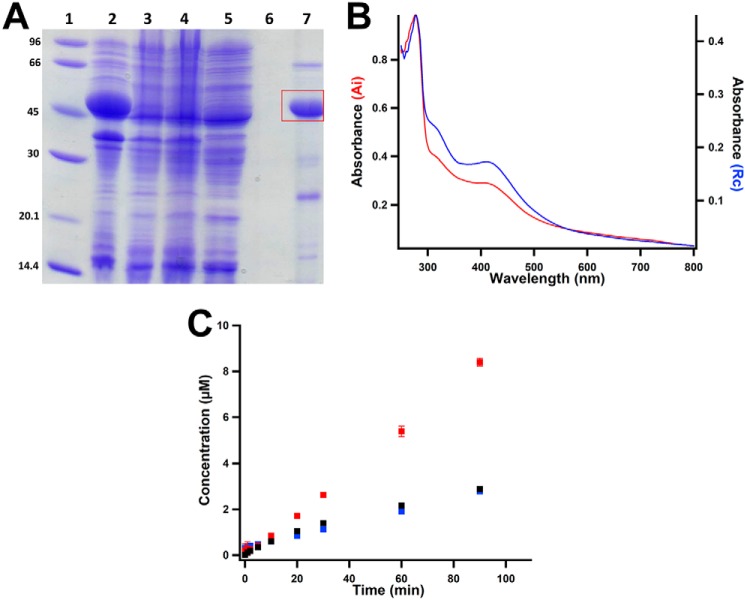
Figure 3.
Characterization of as-purified MaMmp10. A, SDS-PAGE analysis of MaMmp10 purification by immobilized metal-affinity chromatography. Lane 1, molecular mass markers (kDa); lane 2, post-lysis pellet; lane 3, post-lysis supernatant; lane 4, Co-Talon® resin flow-through; lane 5, Co-Talon® start of wash; lane 6, Co-Talon® end of wash; lane 7, eluted MaMmp10. MaMmp10 is denoted by the red box at a theoretical molecular mass of 46.3 kDa. B, UV-visible spectrum of 19.5 μm as-isolated (Ai) MaMmp10 (red trace; A280/A410 = 3.33) and 10.2 μm as-purified (Rc) MaMmp10 (blue trace; A280/A410 = 2.57). C, as-purified MaMmp10 activity with time. 5′-dAH (red squares), SAH (blue squares), and methylated peptide product (black squares) are shown. Reactions were performed in triplicate at 30 °C in a final volume of 200 μl, and they contained the following components: 50 mm HEPES, pH 7.5, 200 mm KCl, 40 μm MaMmp10, 0.5 mm Ti(III) citrate, and 55 μm peptide substrate. Reactions were initiated with 0.5 mm SAM. Error bars indicate the standard deviation of three reactions.