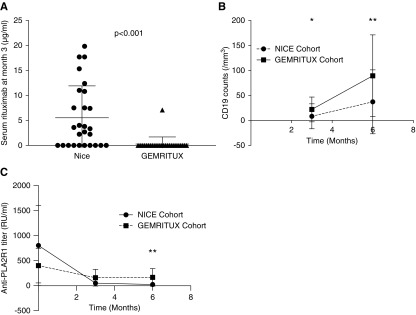
Figure 1.
Temporal changes of circulating intermediate markers of rituximab effect in the NICE and GEMRITUX cohorts. (A) Comparison of serum residual rituximab level at month 3 between the NICE (n=28, two infusions of 1 g at 2-week intervals) and GEMRITUX (n=27, two infusions of 375 mg/m2 at 1-week intervals) cohorts. Note variability of residual rituximab concentrations and lower concentrations in the GEMRITUX participants (P<0.001). Threshold of detection was 2 μg/ml. (B) Evolution of CD19 count at month 3 and month 6 according to the regimens received. *CD19 count was lower in NICE cohort at month 3 (P>0.001). **CD19 count was lower at month 6 in NICE cohort (P=0.03). (C) Evolution of anti-PLA2R1 titer at baseline, month 3, and month 6 according to the regimens received. **Anti-PLA2R1 titer was lower at month 6 in NICE cohort (P>0.001).