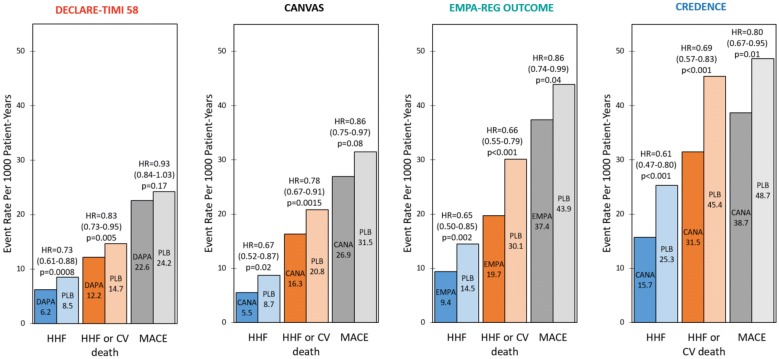
Fig. 2.
Heart failure hospitalization (HHF), HHF and cardiovascular (CV) death, and major adverse cardiovascular event (MACE) event rates per 1000 patients in the Dapagliflozin Effect on CardiovascuLAR Events (DECLARE-TIMI 58), CANagliflozin CardioVascular Assessment Study (CANVAS) Program, Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients–Removing Excess Glucose (EMPA–REG OUTCOME), and Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) trials. Statistical outcomes displayed as hazard ratio, 95% confidence interval, p-value. HR hazard ratio, DAPA dapagliflozin, CANA canagliflozin, EMPA empagliflozin, PLB placebo