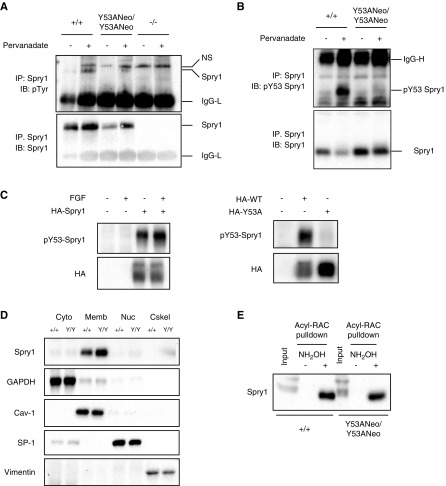
Figure 3.
Characterization of Spry1 Y53A protein. (A, B) Spry1 was immunoprecipitated from pervanadate-stimulated skin fibroblasts of the depicted genotypes, and membranes probed with the indicated antibodies. (C) Validation of phospho-Y53 Spry1 antibody. 293T cells were transfected with empty vector or plasmids coding for hemagglutinin (HA)-tagged wild-type or Y53A mouse Spry1. Cells were stimulated or not with FGF for 10 minutes, lysed, and resolved by SDS-PAGE. Membranes were incubated with the indicated antibodies. Note that overexpression of Sprouty1 in 293T cells leads to constitutive phosphorylation of tyrosine 53. (D) Subcellular fractionation of skin fibroblast extracts indicates that both wild-type and Y53A Sprouty1 proteins localize to the membranous fraction of the cell. GAPDH, caveolin-1, SP-1, and vimentin are markers of cytosolic (Cyto), membranous (Memb), nuclear (Nuc), and cytoskeletal (Cskel) fractions, respectively. (E) Both wild-type and Y53A Sprouty1 are palmitoylated. The presence of a band in the hydroxylamine-treated lysates from skin fibroblasts indicates palmitoylation of the protein (see Methods for details). Acyl-RAC, acyl-resin-assisted capture; IgG-L, low mol wt Ig chains; IgG-H, high mol wt Ig chains; IB, immunoblot; IP, immunoprecipitate; NH2OH, hydroxylamine; NS, nonspecific band; p-, phosphorylated; WT, wild type.