Figure 2.
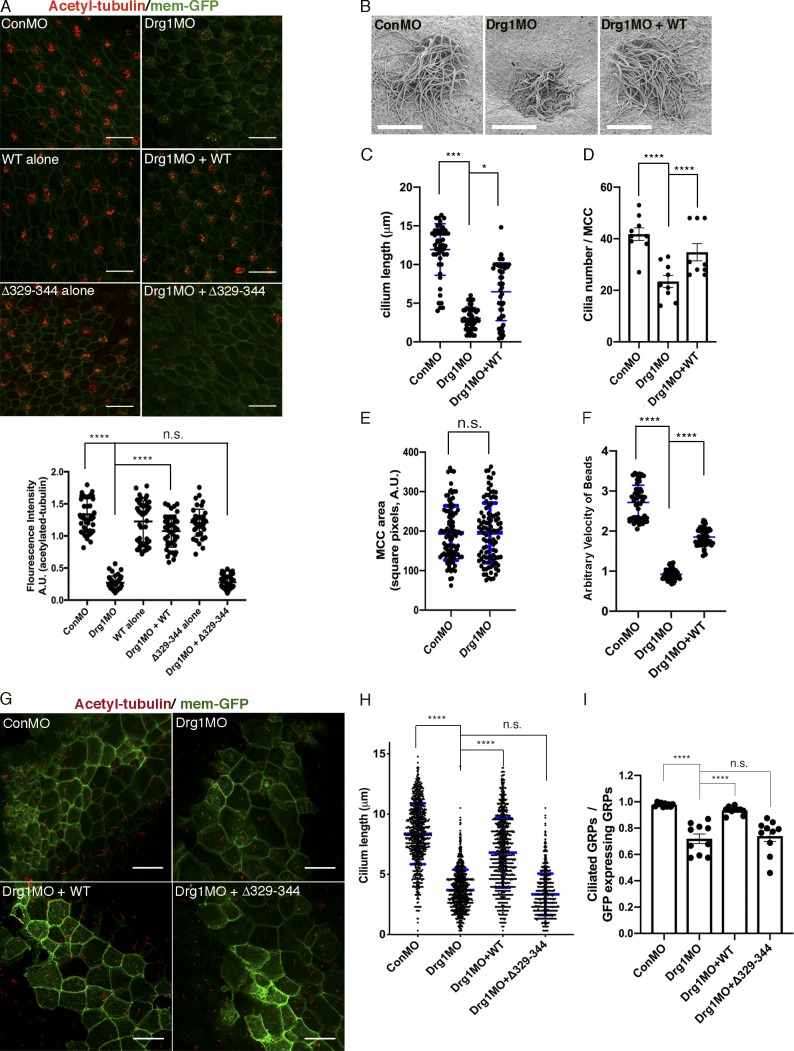
The Drg1–Dvl interaction is important for ciliogenesis in MCCs and the GRP. (A) Drg1 knockdown causes a significant reduction of acetylated tubulin on the epidermis of tadpoles, and the expression of WT Drg1, but not the Δ329–344 mutant, rescues the knockdown phenotype. A cocktail of synthetic mRNAs and morpholinos (7.5 ng each) was injected into both ventral blastomeres at the four-cell stage of embryos. The injected embryos were fixed at stage 27. For immunostaining, anti-acetylated tubulin antibody was used to visualize multicilia (red), and membrane-GFP (green) was used as a tracer. Relative acetylated tubulin intensity is quantified (image n = 45 from 15 embryos for each condition). ****, P < 0.0001, one-way ANOVA; scale bars, 100 µm. Error bars indicate ± SD. (B) Scanning EM of embryonic epidermis confirms Drg1 knockdown causes ciliogenesis defects. 7.5 ng of control and Drg1 morpholinos was injected into both marginal ventral blastomeres at the four-cell stage, and embryos were fixed at stage 31. Scale bars, 12 µm. (C) Quantification of cilia length in MCCs. Data are plotted as the mean, with error bars representing SD. Measured cilia, n > 100; embryos per group, n = 3; ***, P < 0.001; *, P = 0.012, one-way ANOVA. (D) Quantification of cilia number per MCC, n = 10; embryos per group, n = 3; ****, P < 0.0001, one-way ANOVA. Error bars indicate ± SD. (E) The area of epidermal MCCs of stage 27 embryos; MCCs, n = 100; embryos per group, n = 10; two-tailed unpaired t test. Error bars indicate ± SD. (F) Quantification of the fluid flow with green fluorescent beads (Videos 1, 2, and 3). Velocity of five beads along the body axis was manually measured. Measured bead velocity per embryo, n = 5; embryos (stage 27–28) per group, n = 10; stage 27 embryos; ****, P < 0.0001, one-way ANOVA. Error bars indicate ± SD. (G) Drg1 knockdown decreases the length of cilia in the GRP. The indicated mRNAs and MOs were injected to both marginal dorsal blastomeres at the four-cell stage, and embryos were harvested at stage 18. Dissected GRPs were fixed, followed by immunostaining using acetylated tubulin antibody (red) and anti-GFP antibody (green). Mem-GFP is used as a tracer. Scale bars, 30 µm. (H) Quantification of cilia length in the GRP, measured cilia per group; n > 900, dissected GRPs per group; n = 10, ****, P < 0.0001, one-way ANOVA. Error bars indicate ± SD. (I) Quantification of cilia number on GRP; the ratio of the number of ciliated cells expressing GFP to total GRP cells expressing GFP in experimental groups is normalized to the control and expressed as a relative value to the control (set as 1). GRPs per group; n = 10, ****, P < 0.0001, one-way ANOVA. Error bars indicate ± SD. a.u., arbitrary units; n.s., not significant.