Figure 1.
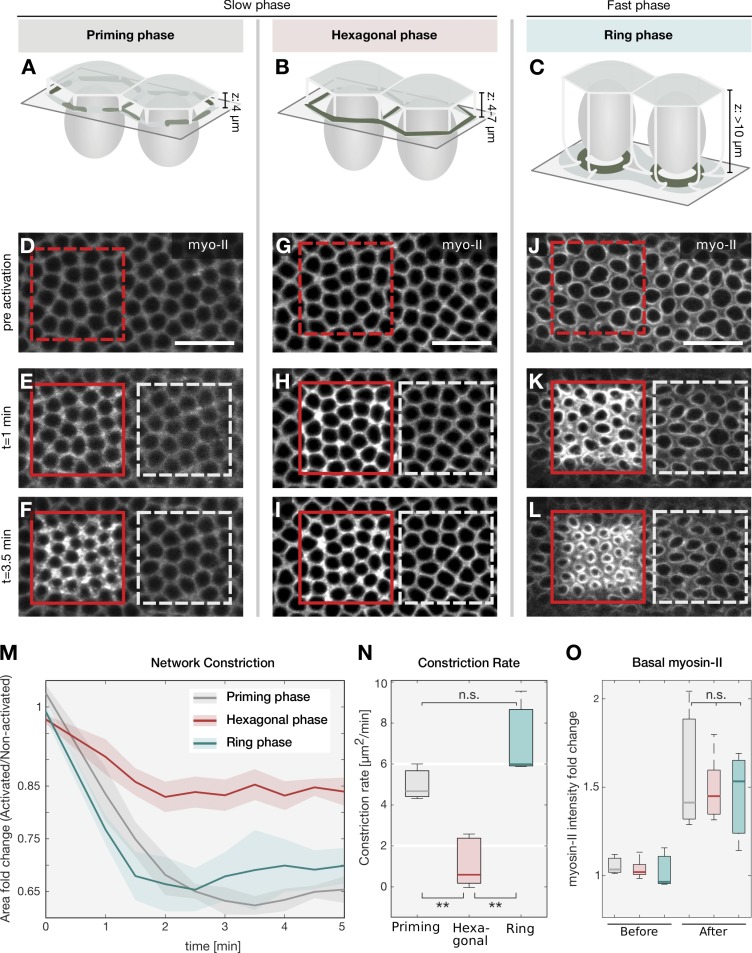
Actomyosin network configurations are differentially sensitive to myosin-II optogenetic stimulation. (A–C) Schematic of the experimental setup. Drosophila embryos express a RhoGEF2 optogenetic system that stimulates myosin-II activity. A subset of cells is photoactivated specifically at the basal surface (gray rectangle) during different stages of cellularization. During cellularization, the plasma membrane ingresses from the apical surface (semitransparent light green) toward the interior of the embryo around the nuclei (gray ovals), separating individual cells. The basal actomyosin network (dark green) assembles during the priming phase (ingression depth [z]: 4 µm; A), acquires a hexagonal configuration during the slow phase (ingression depth [z]: 4–7 µm; B), and breaks down into separated contractile rings during the fast phase (ingression depth [z]: >10 µm; C). (D–L) Confocal sections of Drosophila embryos coexpressing Rho-GEF2-CRY2, CIBN::GFP-pm and the myosin-II probe Sqh::mCh were imaged using a 561-nm laser to visualize the actomyosin network either before photoactivation (D, G, and J), or after photoactivation at the cell base (E, F, H, I, K, and L). Spatially restricted photoactivation was achieved using two-photon excitation (950 nm) in a subset of cells (red square/dashed line: preactivation region; red square/solid line: photoactivated region) during the priming phase (D–F), the hexagonal phase (G–I), or the ring phase (J–L). Upon photoactivation, the myosin-II signal was recorded after 1 min (E, H, and K) and after 3.5 min (F, I, and L). The white dashed squares highlight a region of the same size as of the photoactivation region in the nonactivated part of the embryo. Scale bars in D–L correspond to 20 µm. (M) Quantification of the extent to which the actomyosin network constricted over time upon optogenetic activation of myosin-II during the priming phase (gray, n = 3), hexagonal phase (red, n = 5), and ring phase (green, n = 3). The mean area of individual basal openings within the photoactivated region (number of cells per analyzed embryo: n > 20) was measured and normalized to the nonactivated region (number of cells per analyzed embryo: n > 30) at the respective time point. While during the priming and the ring phase, the actomyosin network constricted to ∼65% of the nonactivated region, during the hexagonal phase it constricted to only 85%. The solid line indicates the mean fold change (area of basal openings in the nonactivated region/activated region), and the semitransparent area represents the SD. (N) Network constriction rate calculated during the first minute after photoactivation. When myosin-II was activated during the priming (gray) or the ring (green) phase, the network constricted with a rate >5 µm/min, compared with a constriction rate of ∼1 µm/min when myosin-II was activated during the hexagonal phase (red). (O) Quantification of myosin-II levels in the photoactivated region before and after photoactivation. Myosin-II levels increased by a factor of ∼1.5 upon light stimulation irrespective of network configuration (gray: priming phase; red: hexagonal phase; green: ring phase) as revealed by one-way ANOVA that did not show any significant differences in myosin-II intensity up-regulation in response to photoactivation during the different stages of cellularization (F[2,8] = 0.16, P = 0.8511). (N and O) In each box plot, the central mark, the bottom, and the top edge indicate the median and the 25th and 75th percentiles, respectively. Whiskers extend to the most extreme data point. The sample numbers are the same as described in M. For all panels, when indicated, significances were estimated using nonpaired two-sample Student’s t test with **, P < 0.01; n.s., not significant.