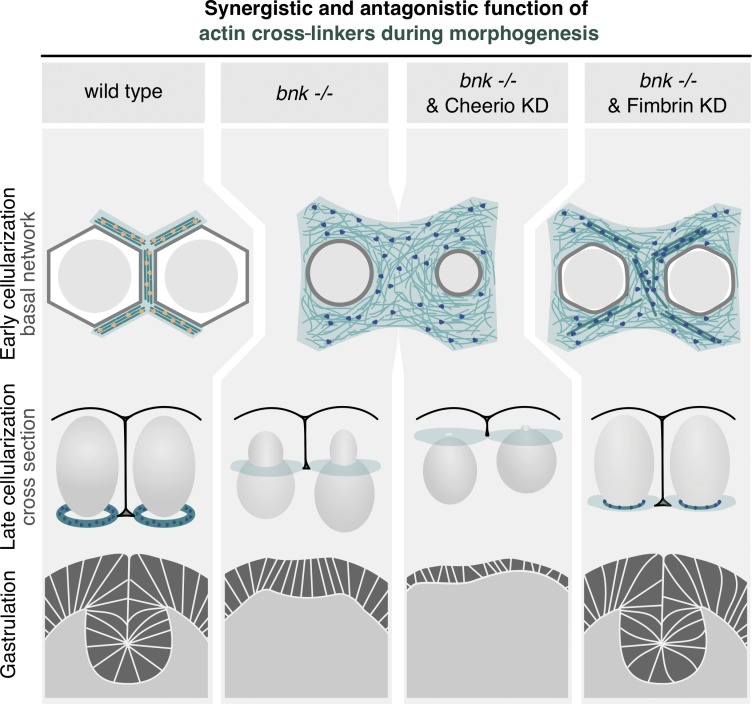
Figure 8.
Model proposing how the synergistic and antagonistic role of actin cross-linkers control the establishment and remodeling of the basal actomyosin network during Drosophila morphogenesis. In a wild-type embryo during early cellularization, Bnk and Cheerio (yellow dots) organize the basal actomyosin network in noncontractile hexagonal arrays of cross-linked actin filaments (green), which are attached to the plasma membrane (gray hexagon) and surround the nuclei (light gray circles). In addition to cross-linking actin filaments, Bnk and Cheerio may ensure attachment of actin filaments to the plasma membrane. During late cellularization, when Bnk is no longer present, the actomyosin hexagonal arrays break down into individual rings that close off the base of the cells in a process that requires Fimbrin cross-linking activity (blue dots). Fimbrin may function to link actin filaments such that transmission of myosin-II forces transforms the hexagonal arrays in ring-like structures. The correct timing of these events promotes formation of correctly shaped epithelial cells and normal gastrulation movements (ventral furrow invagination). In bnk or Cheerio mutant embryos, lack of actin cross-linking results in a meshwork-like organization of actin filaments and premature constriction. Bnk and Cheerio act synergistically, whereas removal of Fimbrin in bnk−/− embryos rescues the premature contraction phenotype and normal embryonic development can proceed, including gastrulation.