Short abstract
Background
MicroRNA (miR)-145-5p is a respiratory disease biomarker, and is upregulated in asthma pathogenesis. However, its underlying mechanisms were unclear, so were investigated in the present study.
Methods
A mouse model of asthma was established by challenge with house dust mite (HDM) extract. An miR-145-5p antagomir was administered nasally and expression of kinesin family member 3A (KIF3A) and miR-145-5p was measured by immunohistochemistry, PCR, and western blot. Eosinophils in lavage fluid and levels of interleukin (IL)-4, IL-5, and IL-13 were quantified. Airway hyper-responsiveness was measured and KIF3A expression was tested following miR-145-5p overexpression or interference in the 16HBE14o- airway epithelial cell line. The effects of miR-145-5p and KIF3A co-transfection in 16HBE14o- cells were examined on cytokine release, epithelial barrier dysfunction, and epithelial repair in HDM-exposed cells.
Results
KIF3A downregulation and miR-145-5p upregulation were noted in airway epithelial cells of HDM-exposed asthmatic mice, while miR-145-5p antagonism significantly improved symptoms. MiR-145-5p promoted the HDM-induced release of chemokines and inflammatory factors and epithelial barrier dysfunction, and suppressed epithelial repair by directly targeting KIF3A.
Conclusion
miR-145-5p influenced HDM-induced epithelial cytokine release and epithelial barrier dysfunction via regulating KIF3 expression. It also affected epithelial repair, exacerbating the HDM-induced T helper 2-type immune response in mice.
Keywords: miR-145-5p, KIF3A, asthma, chemokines, inflammation, epithelial barrier
Introduction
Asthma is a chronic inflammatory airway disorder that leads to symptoms such as coughing, wheezing, and chest tightness. Allergic asthma is characterized by allergen-induced airway inflammation, hyper-responsiveness (AHR), and remodeling.1 The airway epithelium is the first barrier against aeroallergen deposition and plays a central role in the initiation of allergic responses.2–5 The respiratory epithelium is considered to be a central mediator of inflammatory, immune, and regenerative responses to allergens, viruses, and environmental pollutants that contribute to asthma pathogenesis.6 Many allergens, such as the house dust mite (HDM), cause epithelial damage and induce asthma,7,8 whereas pollutants such as ozone also alter the respiratory immune response and cause adverse health effects.9 Alleviating the inflammatory response remains an effective treatment of asthma.
MicroRNAs (miRNAs) are short noncoding RNAs which are important regulators of the posttranscriptional modulation of gene expression.10,11 They regulate a wide range of cellular activities including the inflammatory response.12–14 Because airway inflammation is a major hallmark of asthma, miRNAs may be important therapeutic targets. Indeed, numerous miRNAs have been demonstrated to participate in the initiation and progression of asthma including miR-17, miR-21,15 and miR-33b.16
miR-145-5p was previously found to be significantly increased in the plasma of patients with chronic obstructive pulmonary disease (COPD) and asthma, indicating that plasma miR-145-5p is a specific biomarker of respiratory disease.17 Ozone is associated with numerous adverse health effects and was found to significantly increase the expression of a variety of miRNAs, including miR-145-5p, in human bronchial airways in vivo at a concentration of 0.4 ppm for 2 hours.18 Moreover, in HDM-induced asthma mice models, HDM increased the expression of miR-145-5p, while miR-145-5p inhibition reduced eosinophilic inflammation, mucus hypersecretion, type 2 helper T cell (Th2) cytokine production, and AHR.19 However, although the upregulation of miR-145-5p plays a role in the pathogenesis of asthma, its underlying mechanism remains unclear.
This work aimed to evaluate how miR-145-5p affects asthma progression in an HDM-induced mouse model of asthma. We found that miR-145-5p expression was significantly increased in the airway epithelial tissue sampled from asthmatic mice, whereas expression of its predicted target gene, kinesin family member 3A (KIF3A), was downregulated. miR-145-5p antagonism markedly suppressed the HDM-induced immune response and improved asthma symptoms. In vitro results revealed that miR-145-5p directly targeted the 3′ untranslated region (UTR) of KIF3A, thereby regulating HDM-induced and inflammatory cytokine release by the airway epithelium. Additionally, miR-145-5p promoted HDM-induced epithelial barrier dysfunction and inhibited epithelial repair via KIF3A regulation.
Materials and methods
Mice
Six-week old male specific-pathogen-free (SPF) BALB/c mice weighing 20 to 24 g were purchased from Shanghai Laboratory Animal Center, Chinese Academy of Sciences (Shanghai, China). The mice were kept in a SPF environment (room temperature 24°C, humidity range 40% to 70%, 12 hour light/dark cycle). Sterilized water and food were provided ad libitum. All animal experiments were performed according to guidelines established by the Animal Care and Use Committee. Research was approved by the Ethics Committee of Chongqing Medical University.
Induction of allergic airway disease
All mice were randomly assigned to one of three groups: a control group (1), an HDM+ant-scrambled group (2), and an HDM+ant-miR-145-5p group (3). Mice in groups 2 and 3 were sensitized with 50 µg HDM (Greer Laboratories, Lenoir, NC, USA) resuspended in 50 µL sterile saline intranasally on days 1, 2, and 3. They were then challenged by daily HDM exposure (5 µg/50 µL) on days 14, 15, 16, and 17 to induce allergic airway disease. Mice in group 3 were treated intranasally with 50 µg of miR-145-5pin antagomir and 50 µL sterile saline on day 13 (24 hours before the first HDM re-exposure) and then every second day until they were sacrificed on day 18. Mice in group 2 were treated intranasally with scrambled miR-145-5p synchronously. Mice in group 1 were treated with vehicle sterile endotoxin-free saline at the corresponding time points for sensitization and challenge; they received sterile endotoxin-free saline only when mice from the other two groups were treated with scrambled miR-145-5p or miR-145-5p antagomirs. Mice were sacrificed by cervical dislocation on day 18, 24 hours after their last exposure to HDM.
Antagomir
Target miRNA sequences were downloaded from miRBase. Specific antagomir and scrambled control (ant-scrambled) sequences, identified after a BLAST search against the human genome, were synthesized by GenePharma, Inc. (Shanghai, China). Antagomir sequences were as follows: ant-miR-145-5p, 5′-mAmGmGmGmAUUC CUGG GA AA ACmUmGmGmAmC-3′; scrambled miR: 5′-mAmAmAmAmCCUUUUGACCGAGCmGmUmGmUmU-3′.
AHR measurement
AHR was measured on day 18 as previously described with minor adjustments.20 After mice were anesthetized with 1% pentobarbital sodium (50 mg/kg) via intraperitoneal injection, they were placed in a barometric plethysmographic chamber (Buxco Electronics, Inc., Troy, NY, USA) and administered successively increasing doses of acetylcholine (mg/mL) (0, 6, 12, or 24 mg/mL) via a nebulizer (Buxco Electronics, Inc.) for 3 minutes. Respiratory readings were taken and bronchopulmonary resistance was expressed as RL values.
Cell culture and treatment
The human bronchial epithelial cell line 16HBE14o- (16HBE) was cultured in Eagle’s minimum essential medium (Life Technologies Europe BC, Bleiswijk, the Netherlands) supplemented with 10% fetal calf serum in collagen-coated flasks. Cells were incubated at 37°C with 5% CO2. When 95% to98% confluency was attained, cells were seeded in culture plates at a density of 104 to 105 cells/cm2. After incubation in serum or hormone/growth factor-free medium overnight, cells were treated with 50 µg/mL HDM (Greer Laboratories) and collected via centrifugation 24 hours later. Chemokine levels and inflammatory factors CCL20, CCL2, CCL17, CCL11, granulocyte-macrophage colony-stimulating factor, interleukin (IL)-33, IL-25, and thymic stromal lymphopoietin in the supernatant were measured by enzyme-linked immunosorbent assays (ELISAs) (R&D Systems, Shanghai, China).
RNA extraction and quantitative PCR
Total RNA was extracted using TRIzol reagent (Invitrogen Corp., Carlsbad, CA, USA). A total of 1 µg RNA was reverse-transcribed into cDNA with M-MLV Reverse Transcriptase (Promega, Madison, WI, USA) according to the manufacturer’s protocol. Quantitative reverse transcription (qRT)-PCR was performed with SYBR Premix Ex Taq (Takara Bio Inc., Shiga, Japan) on an ABI 7500 real-time PCR system (Applied Biosystems, Foster City, CA, USA) under the following conditions: 95°C for 40 s, 56°C for 10 s, and 72°C for 50 s, for 35 cycles. The relative expression of genes (miR-145-5p, U6, KIF3A, and GAPDH) was calculated using the 2−ΔΔCt method. Each experiment was performed in duplicate.
Western blot analysis
Cells were suspended and lysed in radioimmunoprecipitation assay buffer (Beyotime, Beijing, China) supplemented with a protease inhibitor cocktail (Sigma-Aldrich, St Louis, MO, USA). Protein extractions were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to a polyvinylidene fluoride membrane (Millipore, Billerica, MA, USA). The membrane was blocked with 5% (w/v) reagent-grade nonfat milk (Cell Signaling Technology®, Danvers, MA, USA) and incubated overnight with primary antibodies at 4°C followed by incubation with an appropriate horseradish peroxidase (HRP)-conjugated secondary antibody for 1 hour at room temperature. Protein bands were visualized using Clarity™ Western ECL substrate (Bio-Rad, Hercules, CA, USA). The protein level was quantified using ImageJ software (ImageJ 1.44, National Institutes of Health, Bethesda, MA, USA) normalized to β-actin. The primary antibodies used were as follows: KIF3A (ab133587, 1:1000, Abcam, Cambridge, MA, USA), E-cadherin (ab76055, 1:1000, Abcam), β-catenin (ab32572, 1:1000, Abcam), occludin (ab167161, 1:100000, Abcam), claudin1 (ab15098, 1:50, Abcam), and β-actin (ab8226, 1:500, Abcam).
Histology and immunohistochemistry
Tissues were fixed overnight with 4% neutral formalin. Tissue sections were stained with hematoxylin and eosin (H&E) for pathological evaluation, and Periodic acid–Schiff (PAS) staining was performed using the PAS staining kit (ab150680, Abcam) according to the manufacturer’s instructions. Tissue sections were also stained with an anti-KIF3A antibody and incubated with an HRP-conjugated secondary antibody (dilution 1:200, Sigma-Aldrich) using standardized immunohistochemical protocols.
Luciferase reporter assay
Luciferase assays were carried out using the Dual-luciferase Reporter Assay System (Promega). Briefly, cells were co-transfected with miR-145-5p mimics or miR-control and pMIR-reporter luciferase vector containing a specific sequence of a wild-type or mutant KIF3A fragment, using Lipofectamine 2000 (Invitrogen). Cells were collected and lysed for luciferase detection 48 hours after transfection. Relative luciferase activity was normalized against Renilla luciferase activity.
Differential cell counts in bronchoalveolar lavage
Bronchoalveolar lavage was performed and total cell numbers in lavage fluid were determined using a Coulter Counter (IG Instrumenten-Gesellschaft AG, Basel, Switzerland). Differential cell counts were performed on cytospins stained with Diff-Quick solution (Dade Behring, Siemens Healthcare Diagnostics, Deerfield, IL, USA). Percentages of eosinophils and neutrophils were determined within a total count of 200 cells per sample.
Cytokine analysis
Chemokine and inflammatory factor levels in cell culture supernatants were measured by ELISA. Baseline levels were set to 1, and mean levels (±SD) are shown.
Electric Cell-Substrate Impedance Sensing (ECIS)
Electrical properties of confluent or wounded epithelium were measured using electric ECIS as described previously.21 Cell adhesion measurements were based on changes in resistance/capacitance to current flow applied at different frequencies (Applied Biophysics, Troy, NY, USA). Cells were inoculated in duplicate at 75 × 103 cells/well in a volume of 400 µL, and resistance/capacitance was measured at 400 and 40,000 Hz. Wounding was performed by electroporation using voltage pulses of 5 V and 40 kHz for 30 s.
Epithelial cell migration assay
Cells were seeded and transfected with miR-145-5p mimics or KIF3A. Seventy-two hours after transfection, they were stained with di-8-ANEPPS (Biotium, Fremont, CA, USA) diluted 1:500 in culture medium, then a p200 pipette tip was used to scratch the plate. Cells were then washed with phosphate-buffered saline and treated with culture media containing di-8-ANEPPS. A Nikon A1Rsi inverted laser scanning confocal microscope, equipped with a motorized x-y stage, Tokai Hit microplate incubator, and Perfect Focus System, was used to document cell migration. Cells were imaged at preselected x-y coordinate points in each well, once every 10 minutes over the course of 16 to 22 hours. Cell migration was analyzed using a spot-tracking algorithm in Imaris software (Bitplane, Zurich, Switzerland).
Statistical analysis
All data are presented as the mean ± SD and were derived from at least three independent experiments. Statistical analysis was performed using SPSS 18.0 (SPSS Inc., Chicago, IL, USA) and GraphPad Prism software (GraphPad Software, Inc., San Diego, CA, USA). All comparisons were deemed statistically significant when P < 0.05.
Results
KIF3A downregulation and miR-145-5p upregulation were detected in asthmatic mouse airway epithelial cells, while miR-145-5p antagonism significantly improved symptoms
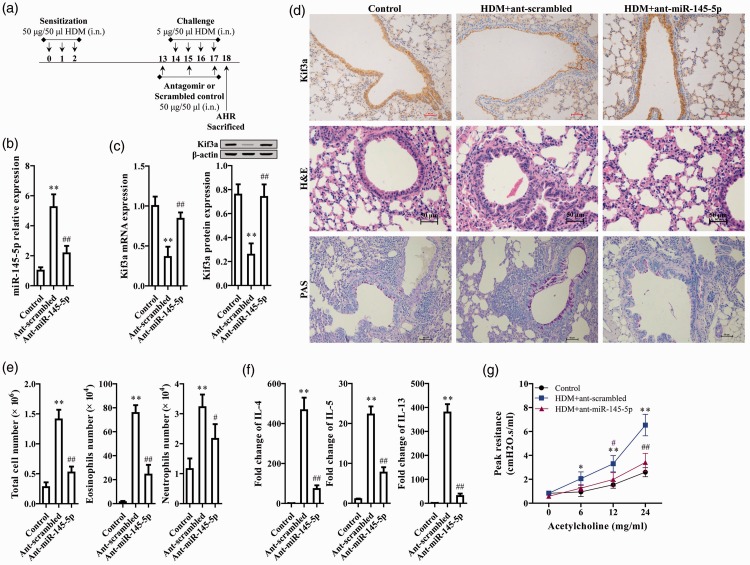
Mice were intranasally treated with HDM (50 µg/50 µL for the first 3 days and 5 µg/50 µL on days 14 to 17) to induce asthma. The miR-145-5p antagomir was administered on days 13, 15, and 17, and mice were sacrificed on day 18. A schematic workflow chart is shown in Figure 1a. HDM markedly upregulated miR-145-5p expression compared with the control, as measured by qRT-PCR, while the miR-145-5p antagomir significantly reduced HDM-induced miR-145-5p upregulation (P<0.01, Figure 1b). In the HDM-induced asthma mice model, KIF3A expression was significantly reduced both at the mRNA and protein level (P<0.01, Figure 1c). Interestingly, the miR-145-5p antagomir significantly reversed HDM-induced downregulation of KIF3A (P<0.01, Figure 1c), indicating that this was mediated by miR-145-5p.
Figure 1.
KIF3A downregulation and miR-145-5p upregulation were detected in airway epithelial cells in HDM-exposed mice with asthma, while miR-145-5p antagonism markedly improved asthma symptoms. (a) Schematic workflow chart for experimental design. (b) miR-145-5p expression was measured by qRT-PCR. (c) KIF3A mRNA and protein expression was determined by qRT-PCR and western blot analysis, respectively. (d) Lung tissue sections were stained with KIF3A immunohistochemistry, H&E, and PAS stains. (e) Total eosinophil and neutrophil numbers. (f) Levels of IL-4, IL-5, and IL-3. (g) Lung resistance is presented as a percentage change over baseline measured in response to inhaled acetylcholine. **P < 0.01, compared with control; ##P < 0.01, #P < 0.05 compared with Ant-scrambled.
Effects of the miR-145-5p antagomir on KIF3A expression and asthma regulation were further evaluated via histological analysis. Immunochemistry staining revealed that KIF3A protein levels were markedly decreased upon HDM induction, but reversed to normal levels upon miR-145-5p antagomir treatment (Figure 1d). HDM exposure promoted inflammatory cell infiltration and higher mucus production in respiratory tissue, as indicated by H&E and PAS staining, respectively (Figure 1d). Pathological characteristics of asthma were largely alleviated upon miR-145-5p antagomir treatment (Figure 1d). HDM exposure significantly increased total inflammatory cell, eosinophil, and neutrophil cell numbers (P<0.01, Figure 1e). Moreover, HDM also markedly elevated cytokine release, including IL-4, IL-5, and IL-13 (P<0.01, Figure 1f). Inhibition of miR-145-5p markedly reduced the HDM-induced inflammatory response (P<0.05, P<0.01, Figure 1e and 1f), as did the miR-145-5p antagomir (P<0.01, Figure 1g). miR-145-5p was thus found to be upregulated and to decrease KIF3A expression in mice with asthma, while its inhibition markedly improved asthmatic symptoms.
miR-145-5p directly regulated KIF3A expression in epithelial cells
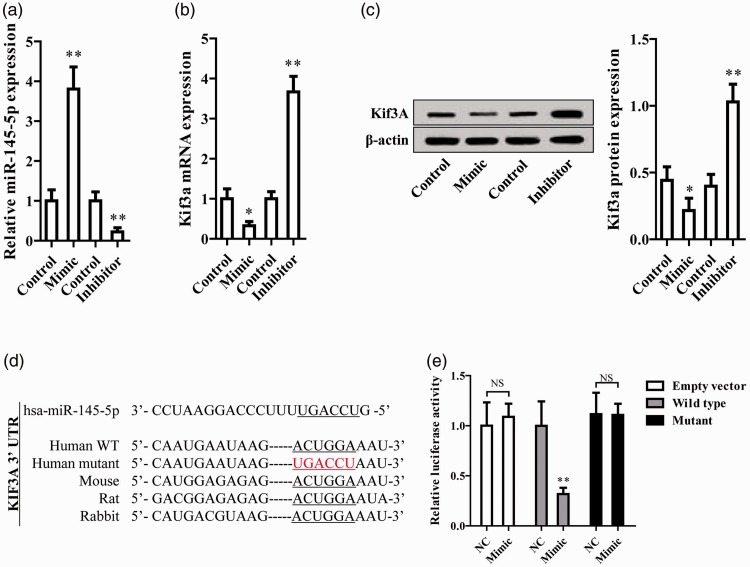
miR-145-5p mimics and inhibitor were transfected into the 16HBE14o- cell line, significantly increasing and inhibiting miR-145-5p expression, respectively, as shown by qRT-PCR (P<0.01, Figure 2a). Interestingly, miR-145-5p mimics significantly reduced KIF3A mRNA expression while the miR-145-5p inhibitor markedly increased KIF3A expression (P<0.05 and P<0.01, respectively, Figure 2b). KIF3A protein expression also decreased after miR-145-5p mimic treatment, and increased upon miR-145-5p inhibitor treatment (Figure 2c).
Figure 2.
miR-145-5p regulates KIF3A expression in epithelial cells by direct targeting. (a, b) miR-145-5p and KIF3A mRNA expression was measured by qRT-PCR. (c) Quantified KIF3A protein expression as determined by western blot. (d) Sequence alignment of miR-145-5p and KIF3A. (e) miR-145-5p reduced the activity of the luciferase reporter with KIF3A wild-type 3′-UTR but not with the mutant 3′-UTR. **P < 0.01, *P < 0.05 compared with control.
The putative KIF3A miR-145-5p target binding sequence and its mutated binding sequence were cloned into a luciferase reporter construct, then transfected into epithelial cells (Figure 2d). miR-145-5p was found to significantly reduce the luciferase activity of the reporter containing the wild-type KIF3A sequence but not that of the mutant sequence (P<0.01, Figure 2e), implying that miR-145-5p directly bound to KIF3A and suppressed its expression.
miR-145-5p promoted the HDM-induced release of chemokines and inflammatory factors via KIF3A regulation
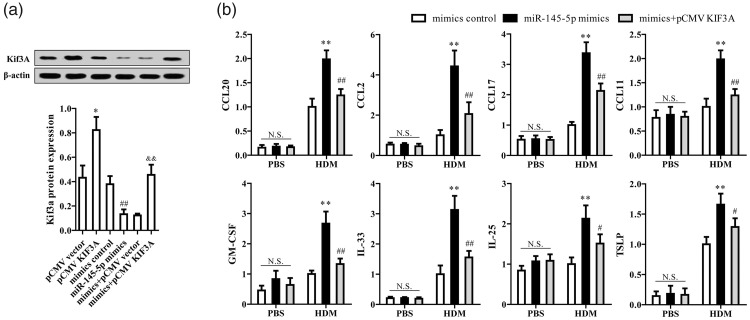
The KIF3A overexpression vector greatly increased its associated protein level and reversed miR-145-5p-mediated reduction of KIF3A (P<0.01, Figure 3a). miR-145-5p mimics significantly escalated the HDM-induced immune response as indicated by the release of chemokines and inflammatory factors, which were markedly reduced by KIF3A overexpression (P<0.01, Figure 3b). miR-145-5p thus promoted HDM-induced release of chemokines and inflammatory factors by suppressing KIF3A.
Figure 3.
miR-145-5p promotes HDM-induced release of chemokines and inflammatory factors via KIF3A regulation. (a) Quantified KIF3A protein expression was measured by western blot analysis. *P < 0.05, compared with pCMV vector; ##P < 0.01 compared with mimic controls; &&P < 0.01 compared with mimics + pCMV vector. (b) Release of CCL20, CCL2, CCL17, CCL11, granulocyte-macrophage colony-stimulating factor, IL-33, IL-25, and thymic stromal lymphopoietin was measured by enzyme-linked immunosorbent assay. **P < 0.01, *P < 0.05 compared with mimic controls; ##P < 0.01, #P < 0.05 compared with miR-145-5p mimics.
miR-145-5p regulated HDM-induced epithelial barrier dysfunction and epithelial repair via KIF3A
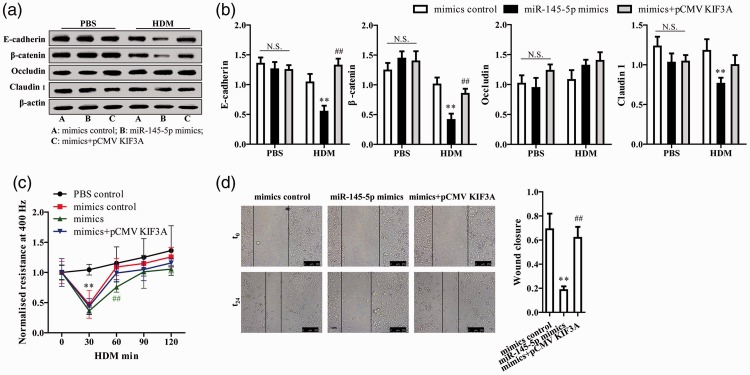
miR-145-5p mimics significantly reduced expression of the cell adhesion-related proteins E-cadherin, β-catenin, and claudin 1 upon HDM exposure; these effects were reversed by KIF3A overexpression (P<0.01, Figure 4a and 4b). Treatment with HDM or miR-145-5p induced a rapid fall in low-frequency (400 Hz) epithelial resistance, but not high-frequency capacitance (Figure 4c), indicating a selective disruption of cell-to-cell adhesion. A reversion to baseline values was noted within 1 hour, although miR-145-5p mimics significantly postponed recovery (P<0.01, Figure 4c). KIF3A overexpression alleviated the effect of miR-145-5p on HDM-induced epithelium barrier dysfunction (Figure 4c).
Figure 4.
miR-145-5p regulates HDM-induced epithelial barrier dysfunction and epithelial repair via KIF3A. (a, b) Epithelial cell adhesion protein expression was measured by western blot analysis and quantified. (c) Absolute resistance values prior to (t = 0) and 30, 60, 90, 120 minutes after HDM exposure are shown. (d) Cell migration was observed by videography for 24 hours and quantified. **P < 0.01, *P < 0.05, compared with mimic controls; ##P < 0.01, #P < 0.05 compared with miR-145-5p mimics.
To assess the role of miR-145-5p and KIF3A in cell proliferation and migration, a ‘scratch’ assay was performed. miR-145-5p mimics were found to significantly suppress the formation of primary cilia and markedly inhibited cell motility and migration (P<0.01, Figure 4d). However, epithelial cells reverted to baseline motility and migration when KIF3A was overexpressed (P<0.01, Figure 4d). miR-145-5p was thus found to inhibit epithelial repair via KIF3A regulation.
Discussion
miRNAs are emerging as important biomarkers and crucial regulators of the pathogenesis of many illnesses. For instance, many miRNAs have been shown to be involved in the initiation and progression of asthma. The decreased expression of epithelial and plasma miR-181b-5p was associated with eosinophilic inflammation of the airway in asthma,22 while the differential expression of miR-1248, miR-26a, Let-7a, and Let-7d were observed in asthmatic patients compared with controls in a study profiling miRNAs.23 Specifically, miR-1248 regulated IL-5 expression, while Let-7 miRNA regulated IL-13 and allergic airway inflammation.5,23 Additionally, aberrant miR-143-3p expression in smooth muscle cells was found to play a role in the pathogenesis of asthma by regulating extracellular matrix protein production.24
In the present study, we found that miR-145-5p was upregulated in the respiratory epithelium of HDM-induced asthmatic mice. Numerous studies have revealed a vital role for miR-145-5p in asthma, with significantly upregulated levels documented in the serum of asthmatic patients, leading to its report as a biomarker for early asthma detection.17 Lacedonia et al.25 also found that miR-145-5p was upregulated in the serum and sputum of patients with asthma. However, the mechanism by which miR-145 affects the progression of asthma has not been widely studied.
The inflammatory response is crucial to the progression and pathology of asthma. Cumulative evidence has shown that miRNA-145-5p is involved in many processes of airway inflammation. For example, Collison et al.26 demonstrated that antagonistic inhibition of miR-145-5p inhibited eosinophilic inflammation, mucus hypersecretion, Th2 cytokine production, and AHR in HDM-induced allergic airway disease. Zhang et al.27 found that miR-145-5p played an important role in macrophage differentiation and polarized activation processes, while Li et al.28 reported that miR-145-5p might partially contribute to the etiology of keloids by affecting several signaling pathways relevant to wound healing. Our study showed that miR-145-5p promoted the HDM-induced release of chemokines and inflammatory factors, leading to an asthmatic state. The administration of a miR-145-5p antagonist remarkably improved asthmatic symptoms by reducing inflammatory cell infiltration and chemokine release, suggesting a potential therapeutic target for treatment of this condition.
We also found that miR-145-5p was involved in the regulation of epithelial barrier dysfunction and repair. The overexpression of miR-145-5p led to reduced cell adhesion-related protein levels, epithelial barrier dysfunction, and suppressed migration and proliferation of epithelial cells, suggesting that miR-145-5p plays a crucial role in the injury and repair processes of airway epithelium. Previously, Chivukula et al.29 showed miR-145-5p to be essential for mouse intestinal epithelial regeneration after injury, while Lee et al.30 observed miR-143/145-expressing clusters in human corneal epithelium, and demonstrated that miR-145-5p regulated the formation of corneal epithelium and maintained epithelial integrity via integrin beta-8.
miRNAs exert their function by inhibiting the translation of their target mRNA gene. Here, we found that miR-145-5p directly bound to 3′-UTR regions of KIF3A, and inhibited its expression at both mRNA and protein levels. KIF3A overexpression partially eliminated miR-145-5p mimic transfection-induced inflammation and chemokine secretion in epithelial cells, as well as epithelial dysfunction and the inhibition of cell migration. KIF3A, on chromosome 5q31, encodes a motor subunit of kinesin-2, and is a human susceptibility gene associated with atopic dermatitis, rhinitis, and asthma.31,32 The KIF3A protein is a component of a trimeric motor complex regulating microtubular function and transport, and is required for the formation and function of motile, nonmotile, and sensory cilia.33,34
The genomic deletion of KIF3A in the mouse is embryonically lethal,31,35,36 and KIF3A polymorphisms were previously shown to be associated with aspirin hypersensitivity in asthma.37 In this study, we found that KIF3A was downregulated in epithelial cells after HDM induction. Moreover, KIF3A overexpression reduced the HDM-induced immune response, as indicated by the release of chemokines and inflammatory factors upon miR-145-5p treatment, and also alleviated the effect of miR-145-5p on HDM-induced epithelial barrier dysfunction and epithelium repair. Previous studies have similarly reported that KIF3A deletion resulted in an enhanced AHR, goblet cell-associated gene expression, and Th2-mediated eosinophilic inflammation.37,38 Additionally, the loss of KIF3A in airway epithelial cells impaired mucociliary clearance and epithelial repair following injury, and enhanced Th2 inflammation, influencing responses to aeroallergens.39
In summary, our study detailed the key roles played by KIF3A in respiratory epithelial cells, as well as in epithelial repair, the innate immune response, and Th2 inflammation-induced airway reactivity. KIF3A is directly targeted by miR-145-5p, which is why it is downregulated in the respiratory epithelial cells of asthma patients. We showed that miR-145-5p influenced HDM-induced epithelial cytokine release and epithelial barrier dysfunction by regulating KIF3A expression and, in turn, affecting epithelial barrier function and repair. These effects led to and exacerbated the HDM-induced Th2-type immune response in mice. The targeting of miR-145-5p or KIF3A are thus potential therapeutic options for asthma treatment.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Anderson GP. Endotyping asthma: new insights into key pathogenic mechanisms in a complex, heterogeneous disease. Lancet 2008; 372: 1107–1119. [DOI] [PubMed] [Google Scholar]
- 2.Holgate ST. Epithelium dysfunction in asthma. J Allergy Clin Immunol 2007; 120: 1233–1244; quiz 1245–1246. [DOI] [PubMed] [Google Scholar]
- 3.Kojima T, Go M, Takano K, et al. Regulation of tight junctions in upper airway epithelium. Biomed Res Int 2013; 2013: 947072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schleimer RP, Kato A, Kern R, et al. Epithelium: at the interface of innate and adaptive immune responses. J Allergy Clin Immunol 2007; 120: 1279–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takano K, Kojima T, Go M, et al. HLA-DR- and CD11c-positive dendritic cells penetrate beyond well-developed epithelial tight junctions in human nasal mucosa of allergic rhinitis. J Histochem Cytochem 2005; 53: 611–619. [DOI] [PubMed] [Google Scholar]
- 6.Lambrecht BN, Hammad H. The airway epithelium in asthma. Nat Med 2012; 18: 684–692. [DOI] [PubMed] [Google Scholar]
- 7.Kauffman HF, Tomee JF, van de Riet MA, et al. Protease-dependent activation of epithelial cells by fungal allergens leads to morphologic changes and cytokine production. J Allergy Clin Immunol 2000; 105: 1185–1193. [DOI] [PubMed] [Google Scholar]
- 8.Tomee JF, van Weissenbruch R, de Monchy JG, et al. Interactions between inhalant allergen extracts and airway epithelial cells: effect on cytokine production and cell detachment. J Allergy Clin Immunol 1998; 102: 75–85. [DOI] [PubMed] [Google Scholar]
- 9.Kim CS, Alexis NE, Rappold AG, et al. Lung function and inflammatory responses in healthy young adults exposed to 0.06 ppm ozone for 6.6 hours. Am J Respir Crit Care Med 2011; 183: 1215–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer 2006; 6: 857–866. [DOI] [PubMed] [Google Scholar]
- 11.Xie CH, Cao YM, Huang Y, et al. Long non-coding RNA TUG1 contributes to tumorigenesis of human osteosarcoma by sponging miR-9-5p and regulating POU2F1 expression. Tumour Biol 2016; 37: 15031–15041. [DOI] [PubMed] [Google Scholar]
- 12.O'Connell RM, Rao DS, Chaudhuri AA, et al. Physiological and pathological roles for microRNAs in the immune system. Nat Rev Immunol 2010; 10: 111–122. [DOI] [PubMed] [Google Scholar]
- 13.Mattes J, Collison A, Foster PS. Emerging role of microRNAs in disease pathogenesis and strategies for therapeutic modulation. Curr Opin Mol Ther 2008; 10: 150–157. [PubMed] [Google Scholar]
- 14.Xiao C, Rajewsky K. MicroRNA control in the immune system: basic principles. Cell 2009; 136: 26–36. [DOI] [PubMed] [Google Scholar]
- 15.Bartel S, Landgrafrauf K, Böck A, et al. Involvement of miRNA-17 and -21 in regulatory T cell (Treg) function in asthma. Eur Respir J 2017; 50: PA2022. [Google Scholar]
- 16.Niu R, Xiao X, Liu B, et al. Inhibition of airway inflammation in a cockroach allergen model of asthma by agonists of miRNA-33b. Sci Rep 2017; 7: 7409. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Wang M, Huang Y, Liang Z, et al. Plasma miRNAs might be promising biomarkers of chronic obstructive pulmonary disease. Clin Respir J 2016; 10: 104–111. [DOI] [PubMed] [Google Scholar]
- 18.Fry RC, Rager JE, Bauer R, et al. Air toxics and epigenetic effects: ozone altered microRNAs in the sputum of human subjects. Am J Physiol Lung Cell Mol Physiol 2014; 306: L1129–L1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collison A, Mattes J, Plank M, et al. Inhibition of house dust mite-induced allergic airways disease by antagonism of microRNA-145 is comparable to glucocorticoid treatment. J Allergy Clin Immunol 2011; 128: 160–167.e4. [DOI] [PubMed] [Google Scholar]
- 20.Hu Y, Dong H, Zou M, et al. TSLP signaling blocking alleviates E-cadherin dysfunction of airway epithelium in a HDM-induced asthma model. Cell Immunol 2017; 315: 56–63. [DOI] [PubMed] [Google Scholar]
- 21.Wegener J, Keese CR, Giaever I. Electric cell-substrate impedance sensing (ECIS) as a noninvasive means to monitor the kinetics of cell spreading to artificial surfaces. Exp Cell Res 2000; 259: 158–166. [DOI] [PubMed] [Google Scholar]
- 22.Huo X, Zhang K, Yi L, et al. Decreased epithelial and plasma miR-181b-5p expression associates with airway eosinophilic inflammation in asthma. Clin Exp Allergy 2016; 46: 1281–1290. [DOI] [PubMed] [Google Scholar]
- 23.Panganiban RP, Pinkerton MH, Maru SY, et al. Differential microRNA epression in asthma and the role of miR-1248 in regulation of IL-5. Am J Clin Exp Immunol 2012; 1: 154–165. [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng W, Yan K, Xie LY, et al. MiR-143-3p controls TGF-β1-induced cell proliferation and extracellular matrix production in airway smooth muscle via negative regulation of the nuclear factor of activated T cells 1. Mol Immunol 2016; 78: 133–139. [DOI] [PubMed] [Google Scholar]
- 25.Lacedonia D, Palladino GP, Foschino-Barbaro MP, et al. Expression profiling of miRNA-145 and miRNA-338 in serum and sputum of patients with COPD, asthma, and asthma-COPD overlap syndrome phenotype. Int J Chron Obstruct Pulmon Dis 2017; 12: 1811–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Collison A, Mattes J, Plank M, et al. Inhibition of house dust mite-induced allergic airways disease by antagonism of microRNA-145 is comparable to glucocorticoid treatment. J Allergy Clin Immunol 2011; 128: 160–167. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, Zhang M, Zhong M, et al. Expression profiles of miRNAs in polarized macrophages. Int J Mol Med 2013; 31: 797–802. [DOI] [PubMed] [Google Scholar]
- 28.Li C, Bai Y, Liu H, et al. Comparative study of microRNA profiling in keloid fibroblast and annotation of differential expressed microRNAs. Acta Biochim Biophys Sin (Shanghai) 2013; 45: 692–699. [DOI] [PubMed] [Google Scholar]
- 29.Chivukula RR, Shi G, Acharya A, et al. An essential mesenchymal function for miR-143/145 in intestinal epithelial regeneration. Cell 2014; 157: 1104–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee KW, Teng Y, Wong HK, et al. MicroRNA-145 regulates human corneal epithelial differentiation. Plos One 2011; 6: e21249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marszalek JR, Ruiz-Lozano P, Roberts E, et al. Situs inversus and embryonic ciliary morphogenesis defects in mouse mutants lacking the KIF3A subunit of kinesin-II. Proc Natl Acad Sci U S A 1999; 96: 5043–5048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Corbit KC, Shyer AE, Dowdle WE, et al. Kif3a constrains beta-catenin-dependent Wnt signalling through dual ciliary and non-ciliary mechanisms. Nat Cell Biol 2008; 10: 70–76. [DOI] [PubMed] [Google Scholar]
- 33.Hirokawa N. Stirring up development with the heterotrimeric kinesin KIF3. Traffic 2000; 1: 29–34. [DOI] [PubMed] [Google Scholar]
- 34.Hirokawa N, Noda Y, Tanaka Y, et al. Kinesin superfamily motor proteins and intracellular transport. Nat Rev Mol Cell Biol 2009; 10: 682–696. [DOI] [PubMed] [Google Scholar]
- 35.Lin F, Hiesberger T, Cordes K, et al. Kidney-specific inactivation of the KIF3A subunit of kinesin-II inhibits renal ciliogenesis and produces polycystic kidney disease. Proc Natl Acad Sci U S A 2003; 100: 5286–5291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takeda S, Yonekawa Y, Tanaka Y, et al. Left-right asymmetry and kinesin superfamily protein KIF3A: new insights in determination of laterality and mesoderm induction by kif3A-/- mice analysis. J Cell Biol 1999; 145: 825–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim JH, Cha JY, Cheong HS, et al. KIF3A, a cilia structural gene on chromosome 5q31, and its polymorphisms show an association with aspirin hypersensitivity in asthma. J Clin Immunol 2011; 31: 112–121. [DOI] [PubMed] [Google Scholar]
- 38.Kovacic MB, Myers JM, Wang N, et al. Identification of KIF3A as a novel candidate gene for childhood asthma using RNA expression and population allelic frequencies differences. PLoS One 2011; 6: e23714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giridhar PV, Bell SM, Sridharan A, et al. Airway epithelial KIF3A regulates Th2 responses to aeroallergens. J Immunol 2016; 197: 4228–4239. [DOI] [PMC free article] [PubMed] [Google Scholar]