Short abstract
Objective
The association between mutations in the serotonin transporter gene-linked polymorphic region (5-HTTLPR) and irritable bowel syndrome (IBS) differs between populations. This meta-analysis was designed to assess the relationship between 5-HTTLPR polymorphisms and IBS in a Chinese population.
Methods
Relevant published studies from PubMed, Embase, Web of Science, the Cochrane Library, and China National Knowledge Infrastructure databases were accessed prior to May 2018. Odds ratios (ORs) with 95% confidence intervals (CIs) were pooled using STATA software.
Results
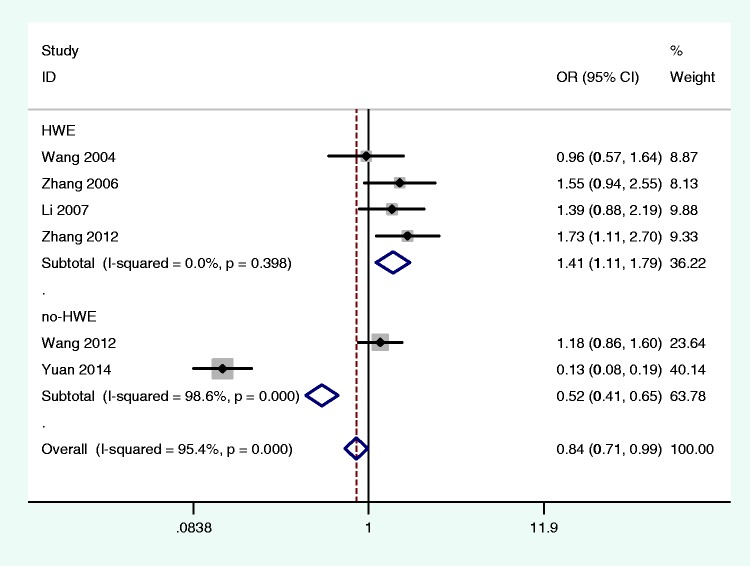
A total of 754 IBS cases and 578 healthy controls in six studies were included in this meta-analysis. Significant results were obtained between 5-HTTLPR polymorphisms and IBS risk among studies with the genotype distribution of controls in Hardy–Weinberg equilibrium (L vs. S, OR = 1.41, 95% CI: 1.11–1.79; LL vs. SS, OR = 2.17, 95% CI: 1.16–4.08; LL vs. LS + SS, OR = 2.29, 95% CI: 1.25–4.20). In subgroup analyses, 5-HTTLPR polymorphisms were significantly associated with increased IBS-C risk in China; however, no risk was observed for IBS-D and IBS-M.
Conclusion
This meta-analysis clearly indicates that 5-HTTLPR polymorphisms are associated with an increased risk of IBS in the Chinese population, especially IBS-C.
Keywords: 5-HTTLPR, polymorphism, irritable bowel syndrome, meta-analysis, Chinese population, IBS-C
Introduction
Irritable bowel syndrome (IBS) is a functional bowel disorder characterized by recurrent abdominal pain occurring at least 1 day per week during the last 3 months and accompanied by a change in bowel habits.1,2 According to different bowel behaviors, IBS can be divided into four subtypes: IBS-C (constipation-predominant), IBS-D (diarrhea-predominant), IBS-M (mixed), and IBS-U (unspecified); IBS-D is the major subtype.3 The high prevalence of IBS in Europe (14%–28%)4 and China (0.82%–11.5%)5,6 adversely impacts the quality of life and increases medical expenses among affected patients.7,8
Although the etiology of IBS remains largely unknown, multiple genetic variations have been shown to affect its development.9–13 Of these, polymorphisms in the serotonin transporter gene-linked polymorphic region (5-HTTLPR) have been most widely evaluated in patients with IBS. 5-HTTLPR short (14 repeats) and long variation (16 repeats) polymorphisms were shown to influence the activity of the serotonin reuptake transporter,14,15 and are very likely to be related to IBS development. However, the potential relationships between 5-HTTLPR polymorphisms and IBS risk in the Chinese population are conflicting.
Meta-analyses based on individual studies with small sample sizes have a lower statistical power. Additionally, a lack of repeatability may also result from inconsistent genetic heritability or lifestyle contexts. Therefore, we conducted the present study to determine the role of 5-HTTLPR polymorphisms in IBS risk in a Chinese population.
Materials and methods
Identification and selection of studies
We evaluated the association between 5-HTTLPR polymorphisms and IBS risk by searching PubMed, Embase, Web of Science, the Cochrane Library, and China National Knowledge Infrastructure databases prior to May 2018 using the following search terms: (“irritable bowel syndrome” or “IBS”) and (“serotonin” or “5-hydroxytryptamine” or “5-HT”) and “polymorphism” and (“Chinese” or “China”). The search was not restricted by language or publication status. At least two independent reviewers screened the potentially relevant articles, and disagreements were resolved by discussion or input from a third reviewer if needed.
The inclusion criteria were as follows: (1) studies examining the relationship between 5-HTTLPR polymorphisms and IBS risk; (2) a case–control study design; (3) sufficient data regarding genotype frequency; and (3) Chinese ethnicity. The exclusion criteria were as follows: (1) overlapping literature; (2) unextractable data; (3) study design other than case–control; and (4) abstract or review article types.
Data extraction
Potentially relevant studies and extracted data from identified publications, including the first author’s name, publication year, geographic area, source of controls, sample size, and available genotype information from 5-HTTLPR polymorphisms, were screened by two investigators. We studied the titles and abstracts for each retrieved document first, then read the full papers if the titles and abstracts did not determine if the study met the inclusion criteria. Data discrepancies in extraction were resolved by discussion between the two investigators. In this meta-analysis, the quality of individual studies was assessed according to the nine-star Newcastle–Ottawa Scale.16
Statistical analysis
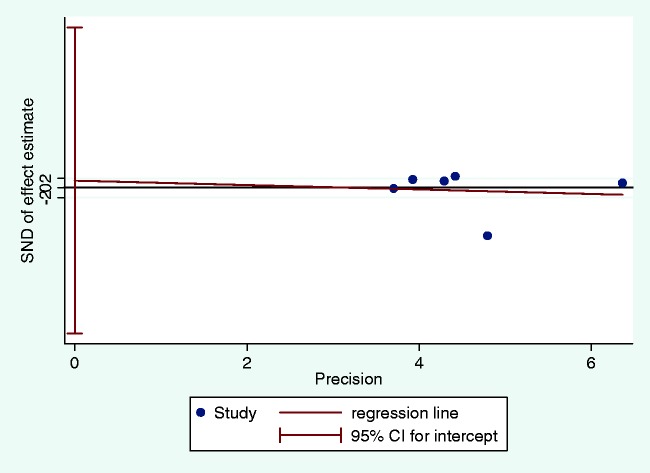
Odds ratios (ORs) with 95% confidence intervals (CIs) were used to evaluate the strength of association between 5-HTTLPR polymorphisms and IBS risk to the model. L versus S, LL versus SS, LL versus (LS + SS), and (LL + LS) versus SS were examined for IBS risk. The comprehensive test of heterogeneity, as well as the Hardy–Weinberg equilibrium (HWE) in controls, was tested by I-squared based on Q and the df.17 When Pheterogeneity < 0.1 or I2 > 50%, a random effects meta-analysis model was applied to estimate the pooled ORs; otherwise the fixed effects model was adopted. The entire calculated ORs were analyzed by the Z-test. Both effects models of each pooled OR were computed for the sensitivity assay. The funnel plot was used to assess potential publication bias and Egger’s test was applied to evaluate funnel plot asymmetry. We also performed a subgroup investigation according to geographic area, IBS subtype, and HWE in controls to evaluate the relationship between 5-HTTLPR and IBS risk. All statistical analyses were performed using Stata version 12 (StataCorp LP, College Station, TX, USA), and P < 0.05 was considered significant.
Results
Research characteristics
Three hundred sixty-seven publications that assessed the relationship between 5-HTTLPR polymorphisms and IBS were identified. Six studies18–23 met the inclusion criteria and were used in this meta-analysis. The publication years included for this meta-analysis ranged from 2004 to 2014. Figure 1 reveals the detailed screening information. A total of 754 IBS cases and 578 healthy controls were included in the current study, which assessed the relationship between 5-HTTLPR polymorphisms and IBS risk among the Chinese population. The main characteristics of the six studies are listed in Table 1.
Figure 1.
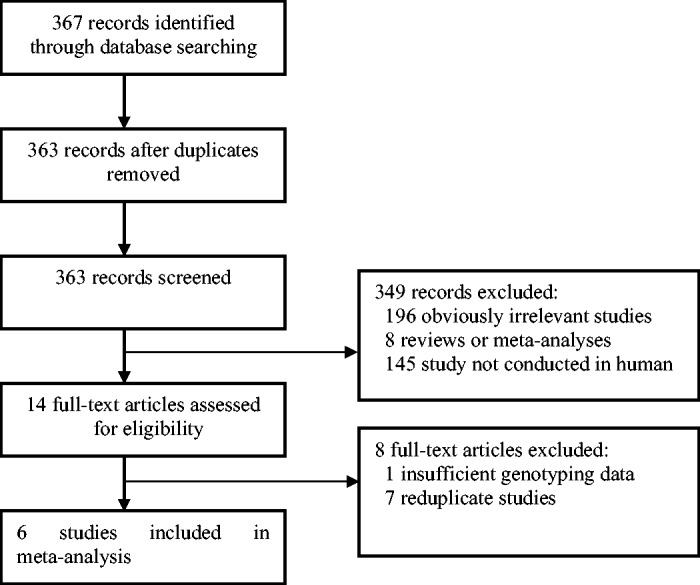
Flow diagram of the literature search.
Table 1.
Characteristics of studies included in the meta-analysis.
| Reference | Geographic area | IBS subtype | Case number | Control number | Cases |
Controls |
HWE |
Quality score | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SS | LS | LL | SS | LS | LL | χ² | P | ||||||
| Wang 2004 | Tianjin | IBS-C, IBS-D, IBS-M | 78 | 44 | 21 | 25 | 32 | 10 | 17 | 17 | 1.89 | 0.169 | 7 |
| Zhang 2006 | Fujian | IBS-C, IBS-D, IBS-M | 145 | 48 | 64 | 49 | 32 | 24 | 20 | 4 | 0.00 | 0.954 | 8 |
| Li 2007 | Guangzhou | IBS-C, IBS-D, IBS-M | 87 | 96 | 46 | 27 | 14 | 55 | 34 | 7 | 0.30 | 0.586 | 8 |
| Wang 2012 | Tianjin | IBS-C, IBS-D, IBS-M | 254 | 120 | 97 | 67 | 90 | 49 | 35 | 36 | 20.15 | 0.000 | 8 |
| Zhang 2012 | Jiangxi | IBS-C | 84 | 103 | 43 | 20 | 21 | 59 | 36 | 8 | 0.56 | 0.453 | 8 |
| Yuan 2014 | Yunnan | IBS-C, IBS-D, IBS-M | 106 | 167 | 45 | 31 | 30 | 20 | 8 | 139 | 108.32 | 0.000 | 8 |
HWE, Hardy–Weinberg equilibrium; IBS, irritable bowel syndrome; IBS-C, constipation-predominant; IBS-D, diarrhea-predominant; IBS-M, mixed.
Overall analysis
No significant associations were observed between 5-HTTLPR and IBS risk among the four models (L vs. S, OR = 0.90, 95% CI: 0.40–2.02; LL vs. SS, OR = 1.15, 95% CI: 0.36–3.71; LL vs. LS + SS, OR = 1.19, 95% CI: 0.34–4.14; LL + LS vs. SS, OR = 0.83, 95% CI: 0.45–1.52; Table 2). There was significant heterogeneity among the tested models (P < 0.05). Sensitivity analysis indicated consistent results for the fixed and random effects models. When evaluating publication bias, the shape of the funnel plot revealed obvious asymmetry (Figure 3). However, Egger’s test indicated that there was no evidence of obvious publication bias in all included studies (t = 0.13, P = 0.900, Figure 4).
Table 2.
Association of the 5-HTTLPR polymorphism with IBS susceptibility.
| Analysis model | n | ORr (95% CI) | ORf (95% CI) | Ph |
|---|---|---|---|---|
| L vs. S | ||||
| Total analysis | 6 | 0.90 (0.40–2.02) | 0.84 (0.71–0.99) | 0.000 |
| North China | 2 | 1.12 (0.86–1.46) | 1.12 (0.86–1.46) | 0.523 |
| South China | 4 | 0.83 (0.22–3.06) | 0.70 (0.57–0.87) | 0.000 |
| In HWE | 4 | 1.41 (1.11–1.79) | 1.41 (1.11–1.79) | 0.398 |
| IBS-C | 5 | 1.84 (1.48–2.30) | 1.84 (1.48–2.30) | 0.740 |
| IBS-D | 4 | 0.87 (0.67–1.1.4) | 0.87 (0.67–1.1.4) | 0.678 |
| IBS-M | 4 | 1.05 (0.67–1.65) | 1.07 (0.78–1.48) | 0.142 |
| LL vs. SS | ||||
| Total analysis | 6 | 1.15 (0.36–3.71) | 0.87 (0.66–1.16) | 0.000 |
| North China | 2 | 1.17 (0.74–1.84) | 1.17 (0.74–1.84) | 0.5360 |
| South China | 4 | 1.23 (0.16–9.12) | 0.72 (0.50–1.03) | 0.000 |
| In HWE | 4 | 2.17 (1.16–4.08) | 2.20 (1.36–3.55) | 0.016 |
| IBS-C | 5 | 2.88 (1.90–4.39) | 2.90 (1.93–4.35) | 0.391 |
| IBS-D | 4 | 0.83 (0.51–1.35) | 0.83 (0.51–1.35) | 0.896 |
| IBS-M | 4 | 1.12 (0.44–2.84) | 1.15 (0.63–2.12) | 0.152 |
| LL vs. LS+SS | ||||
| Total analysis | 6 | 1.19 (0.34–4.14) | 0.80 (0.63–1.03) | 0.000 |
| North China | 2 | 1.23 (0.83–1.83) | 1.23 (0.83–1.83) | 0.745 |
| South China | 4 | 1.22 (0.13–10.98) | 0.60 (0.43–0.83) | 0.000 |
| In HWE | 4 | 2.29 (1.25–4.20) | 2.24 (1.44–3.47) | 0.008 |
| IBS-C | 5 | 3.00 (2.07–4.35) | 3.04 (2.10–4.39) | 0.639 |
| IBS-D | 4 | 0.91 (0.58–1.41) | 0.91 (0.58–1.41) | 0.935 |
| IBS-M | 4 | 0.98 (0.38–2.49) | 0.97 (0.56–1.67) | 0.095 |
| LL+LS vs. SS | ||||
| Total analysis | 6 | 0.83 (0.45–1.52) | 0.85 (0.67–1.07) | 0.000 |
| North China | 2 | 1.04 (0.70–1.55) | 1.04 (0.70–1.54) | 0.498 |
| South China | 4 | 0.77 (0.30–1.57) | 0.76 (0.57–1.01) | 0.000 |
| In HWE | 4 | 1.17 (0.85–1.62) | 1.17 (0.85–1.61) | 0.825 |
| IBS-C | 5 | 1.43 (1.04–1.96) | 1.44 (1.06–1.96) | 0.391 |
| IBS-D | 4 | 0.82 (0.57–1.19) | 0.82 (0.57–1.19) | 0.610 |
| IBS-M | 4 | 1.18 (0.75–1.85) | 1.18 (0.75–1.85) | 0.716 |
5-HTTLPR, serotonin transporter gene-linked polymorphic region; IBS, irritable bowel syndrome; ORr, Odds ratio for random effects model; ORf, Odds ratio for fixed effects model; CI, confidence interval; Ph, P value for heterogeneity test; South China including Fujian, Guangzhou, Jiangxi, Yunnan; HWE, Hardy–Weinberg equilibrium.
Figure 3.
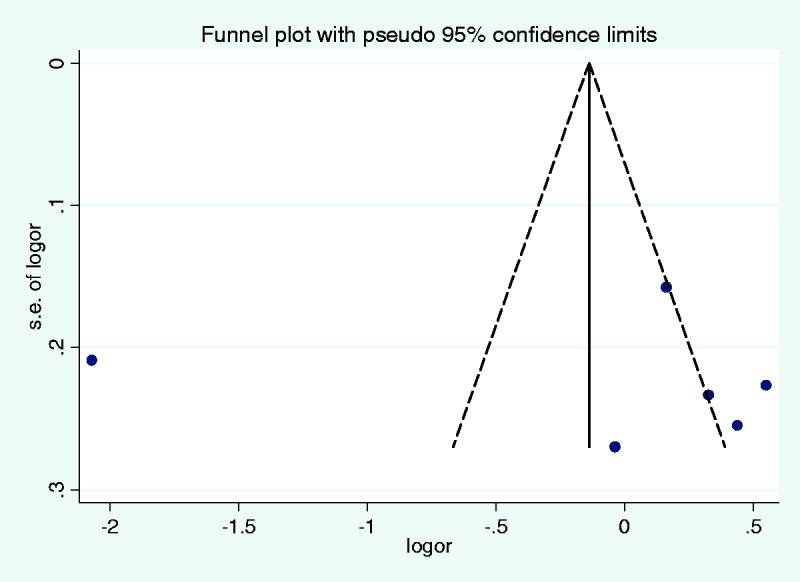
Publication bias assessment with Begg’s funnel plot.
Figure 4.
Egger’s linear regression.
Subgroup analysis
Subgroups were designated according to geographic area and HWE in controls. The data indicated that 5-HTTLPR polymorphisms were not related to IBS risk among populations from north China and south China. Positive associations among studies with controls in HWE in three analysis models were demonstrated (L vs. S, OR = 1.41, 95% CI: 1.11–1.79; LL vs. SS, OR = 2.17, 95% CI: 1.16–4.08; LL vs. LS + SS, OR = 2.29, 95% CI: 1.25–4.20; Figure 2). Next, IBS-C, IBS-D, and IBS-M subgroups were analyzed from the data in five studies. The L allele and LL genotype were significantly correlated with increased risk of IBS-C in China; however, no associations existed between 5-HTTLPR polymorphisms and the risk of IBS-D or IBS-M (Table 2).
Figure 2.
Forest plots of all selected studies about the association between 5-HTTLPR polymorphisms on IBS risk in a Chinese population under the allele model. 5-HTTLPR: serotonin transporter gene-linked polymorphic region; IBS: irritable bowel syndrome.
Discussion
A number of studies have been published to analyze the relationship between 5-HTTLPR polymorphisms and risk of IBS; however, no comprehensive definitive conclusion has been established. Based on a previous meta-analysis,24 no association was detected between 5-HTTLPR polymorphisms and IBS, but mainstream patients among the Asian population were shown to be short allele homozygous (64%). Subgroup analysis based on participant region or population showed a reduced 5-HTTLPR polymorphism effect on IBS risk in Americans and Asians.25 Individual studies are capable of generating diverse results reflecting regional variability and individuals in different populations, or the limited number of cases studied in each analysis. Additionally, unique cultures and lifestyles of diverse ethnic groups may lead to different genetic traits. To reduce these effects, we conducted the current meta-analysis to further survey the relationship between 5-HTTLPR polymorphisms and IBS in the Chinese population.
The current study consisted of six investigations, including 754 IBS cases and 578 healthy controls. The overall analysis indicated that 5-HTTLPR polymorphisms were not associated with IBS risk among the Chinese population. Considering the effect of geographic background, IBS subtype, and HWE in controls on the results, we also performed subgroup analysis with respect to these factors. Positive relationships between 5-HTTLPR polymorphisms and IBS risk were also found among studies with controls in HWE. Furthermore, the L allele and LL genotype were significantly associated with increased IBS-C risk in China. No associations between 5-HTTLPR polymorphisms and the risk of IBS-D or IBS-M were shown. Our results are consistent with the previously published meta-analysis conducted by Zhang et al.,26 which indicated that the association between 5-HTTLPR with IBS-C is population-dependent and that a positive relationship only exists in the east Asian population, not in the Caucasian population.
One important 5-HTTLPR polymorphism feature is that the variation may be significant between different groups and/or races.27 A strength of this study is that the association between 5-HTTLPR mutations and IBS risk is pooled only in the Chinese population. Heterogeneity is fairly common in meta-analyses among genetic association studies. When considering all of the eligible data, we found heterogeneity between studies. Although the heterogeneity was effectively decreased after subgroup analysis by geographic area, IBS subtype, and HWE in controls, there are some limitations in our meta-analysis that require discussion. First, only studies published in English and Chinese languages were included, and related articles published in other languages and indexed in other databases were excluded. Second, we could not perform other subgroup analyses, such as those based on age, gender, and duration of disease because of data limitation in original papers. Finally, the pathogenesis of IBS is considered multifactorial so a single genetic variant may not predict the risk of IBS.
Conclusion
The results of this meta-analysis clearly showed a positive correlation between 5-HTTLPR polymorphisms and the increased risk of IBS in a Chinese population, especially IBS-C. However, because of limitations in this meta-analysis, further studies with different backgrounds are required to validate this association and to investigate potential gene–gene and gene–environment interactions between 5-HTTLPR polymorphisms and IBS susceptibility.
Abbreviations
5-HTTLPR: serotonin transporter gene linked polymorphic region; IBS: irritable bowel syndrome; OR: odds ratio; CI: confidence interval; HWE: Hardy–Weinberg equilibrium.
Authors’ contributions
Bianfang YU1, Qinggang LI, and Xin DONG designed, collected, and analyzed the data. Zhan-bo Jia and Li-ping Wang drafted the manuscript and approved submission.
Availability of data and materials
All data are included in the study.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Ethics approval and consent to participate
Not applicable.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Chang JY, Talley NJ. Current and emerging therapies in irritable bowel syndrome: from pathophysiology to treatment. Trends Pharmacol Sci 2010; 31: 326–334. [DOI] [PubMed] [Google Scholar]
- 2.Longstreth GF, Thompson WG, Chey WD, et al. Functional bowel disorders. Gastroenterology 2006; 130: 1480–1491. [DOI] [PubMed] [Google Scholar]
- 3.Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol 2012; 10: 712–721. [DOI] [PubMed] [Google Scholar]
- 4.Rusu F, Dumitrascu DL. Epidemiology of irritable bowel syndrome in the former communist countries from Eastern Europe: a systematic review. Clujul Medical 2015; 88: 146–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pan G, Lu S, Ke M, et al. [An epidemiologic study of irritable bowel syndrome in Beijing - a stratified randomized study by clustering sampling]. Zhonghua Liu Xing Bing Xue Za Zhi 2000; 21: 26–29. [PubMed] [Google Scholar]
- 6.Xiong LS, Chen MH, Chen HX, et al. A population-based epidemiologic study of irritable bowel syndrome in Guangdong province. Zhonghua Yi Xue Za Zhi 2004; 84: 278–281. [PubMed] [Google Scholar]
- 7.Simrén M, Svedlund J, Posserud I, et al. Health-related quality of life in patients attending a gastroenterology outpatient clinic: functional disorders versus organic diseases. Clin Gastroenterol Hepatol 2006; 4: 187–195. [DOI] [PubMed] [Google Scholar]
- 8.Katsumata R, Shiotani A, Murao T, et al. The TPH1 rs211105 gene polymorphism affects abdominal symptoms and quality of life of diarrhea-predominant irritable bowel syndrome. J Clin Biochem Nutr 2018; 62: 270–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu Y, Zheng G, Hu Z. Association between SERT insertion/deletion polymorphism and the risk of irritable bowel syndrome: a meta-analysis based on 7039 subjects. Gene 2018; 679: 133–137. [DOI] [PubMed] [Google Scholar]
- 10.Han CJ, Kohen R, Jun S, et al. COMT Val158Met polymorphism and symptom improvement following a cognitively focused intervention for irritable bowel syndrome. Nurs Res 2017; 66: 75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han CJ, Jarrett ME, Cain KC, et al. Association of fatigue with TPH2 genetic polymorphisms in women with irritable bowel syndrome. Biol Res Nurs 2019; 21: 72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun MH, Sun LQ, Guo GL, et al. Tumour necrosis factor-α gene -308 G > A and -238 G > A polymorphisms are associated with susceptibility to irritable bowel syndrome and drug efficacy in children. J Clin Pharm Ther 2019; 44: 180–187. [DOI] [PubMed] [Google Scholar]
- 13.Grzesiak M, Beszłej JA, Waszczuk E, et al. Serotonin-related gene variants in patients with irritable bowel syndrome and depressive or anxiety disorders. Gastroenterol Res Pract 2017; 2017: 4290430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heils A, Teufel A, Petri S, et al. Allelic variation of human serotonin transporter gene expression. J Neurochem 1996; 66: 2621–2624. [DOI] [PubMed] [Google Scholar]
- 15.Yeo A, Boyd P, Lumsden S, et al. Association between a functional polymorphism in the serotonin transporter gene and diarrhoea predominant irritable bowel syndrome in women. Gut 2004; 53: 1452–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wells GA, Shea B, O’Connell D, et al. The Newcastle–Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. [Available from: http: //www.ohri.ca/programs/clinical_epidemiology/oxford.asp]
- 17.Hoaglin DC. Assessment of heterogeneity in meta-analyses. JAMA 2014; 312: 2286–2287. [DOI] [PubMed] [Google Scholar]
- 18.Wang BM, Wang YM, Zhang WM, et al. Serotonin transporter gene polymorphism in irritable bowel syndrome. Zhonghua Nei Ke Za Zhi 2004; 43: 439–441. (article in Chinese) [PubMed] [Google Scholar]
- 19.Zhang XM, Lin ZH. Relationship between serotonin transporter gene polymorphism and irritable bowel syndrome. Shijie Huaren Xiaohua Zazhi 2006; 14: 1790–1794. (article in Chinese) [Google Scholar]
- 20.Li Y, Nie Y, Xie J, et al. The association of serotonin transporter genetic polymorphisms and irritable bowel syndrome and its influence on tegaserod treatment in Chinese patients. Dig Dis Sci 2007; 52: 2942–2949. [DOI] [PubMed] [Google Scholar]
- 21.Wang YM, Chang Y, Chang YY, et al. Serotonin transporter gene promoter region polymorphisms and serotonin transporter expression in the colonic mucosa of irritable bowel syndrome patients. Neurogastroenterol Motil 2012; 24: 560–565. [DOI] [PubMed] [Google Scholar]
- 22.Zhang ZX, Xie J, Huang CB, et al. Serotonin transporter polymorphism in constipation predominant irritable bowel syndrome and its influence on clinical effect of tegaserod. Shandong Yiyao 2012; 52: 16–18. (article in Chinese) [Google Scholar]
- 23.Yuan J, Kang C, Wang M, et al. Association study of serotonin transporter SLC6A4 gene with Chinese Han irritable bowel syndrome. PLoS One 2014; 9: e84414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Kerkhoven LA, Laheij RJ, Jansen JB. Meta-analysis: a functional polymorphism in the gene encoding for activity of the serotonin transporter protein is not associated with the irritable bowel syndrome. Aliment Pharmacol Ther 2007; 26: 979–986. [DOI] [PubMed] [Google Scholar]
- 25.Areeshi MY, Haque S, Panda AK, et al. A serotonin transporter gene (SLC6A4) polymorphism is associated with reduced risk of irritable bowel syndrome in American and Asian Population: a meta-analysis. PLoS One 2013; 8: e75567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang ZF, Duan ZJ, Wang LX, et al. The serotonin transporter gene polymorphism (5-HTTLPR) and irritable bowel syndrome: a meta-analysis of 25 studies. BMC Gastroenterol 2014; 14: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Butovskaya ML, Butovskaya PR, Vasilyev VA, et al. Serotonergic gene polymorphisms (5-HTTLPR, 5HTR1A, 5HTR2A), and population differences in aggression: traditional (Hadza and Datoga) and industrial (Russians) populations compared. J Physiol Anthropol 2018; 37: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are included in the study.