Abstract
Objective:
The objective of the study is to test theoretical intervention fidelity and feasibility of MOVING ON, a self-directed, home-based, randomized controlled trial to increase exercise outcome expectations (OEs) (what one expects to obtain or avoid as a result of a behavior or lack thereof), among breast cancer survivors.
Method:
Stage Ia to IIb survivors (n = 60) were given the MOVING ON intervention or control booklet. Data were collected through online surveys and an accelerometer at baseline, 4, 8, and 12 weeks postintervention. Fidelity was measured by questions assessing participant perceptions of MOVING ON (score ≥2) and direction of intervention effects. Feasibility was measured by recruitment rate (target of 60 participants in 6 months), retention (total attrition <17%), and acquisition of accelerometer data (% ≥subjective exercise data obtained). Analyses consisted of descriptive statistics, mixed models, and content analysis.
Results:
Fidelity met a priori criteria (mean = 3.31, SD = 0.87). Outcome expectations increased 0.01 points, and weekly steps increased by 970 every 4 weeks in the intervention arm compared to the control arm. All effect sizes were small, ranging from 0.01 to 0.09. Target enrollment, achieved in 17 weeks, met a priori feasibility criteria. Retention (66%) and accelerometer data acquisition (60%) (compared to 73% of subjective exercise data) did not.
Conclusion:
MOVING ON influenced OEs as intended and was well received by participants. A fully powered study, of this low-cost, easy-to-implement intervention, is warranted. Intervention and measurement strategies used in MOVING ON can be incorporated in any study targeting OEs as a mediator of exercise or collecting exercise data with an accelerometer.
Keywords: behavior change, breast cancer, cancer, cancer survivor, exercise, outcome expectations, physical activity, survivorship
1 |. BACKGROUND
Cancer survivors are motivated to engage in health behaviors that they believe will improve their long-term outcomes and quality of life.1 Regular aerobic exercise is 1 such behavior that they may benefit from; it is associated with improved survival and increases quality of life for breast cancer survivors.2 However, only 16% to 37% 3,4 of the 3.1 million breast cancer survivors in the United States adhere to the minimum recommended 150 weekly minutes of moderate-intensity aerobic exercise.5,6 Further, among survivors who exercised regularly prediagnosis, exercise levels decrease during and after adjuvant therapy.7,8
One possible explanation for poor exercise adherence among breast cancer survivors is that they have low exercise outcome expectations (OEs). 9 Outcome expectations refer to people’s beliefs about an (in) action leading to an outcome.10 Dimensions of OEs include (1) accessibility—the frequency with which outcome(s) are considered; (2) certainty—perceived probability outcome(s) will occur; and (3) importance—value placed on the outcome(s).11–13 According to several health behavior change theories, increased beliefs that exercise will produce benefits (ie, having high OEs) lead to behavior change.10,14,15 Among noncancer populations, individuals who expect more positive and less negative outcomes of exercise have stronger intentions to exercise and exercise more.16,17
Effective strategies to increase exercise OEs among breast cancer survivors are not well established. Interventions that included OEs, along with other social cognitive predictors of exercise, have increased exercise among breast cancer survivors.18–20 However, the extent to which these interventions effectively increased OEs is not clear because direct effects on OEs were not reported,19,20 or were found negative.18 Additionally, prior interventions have not included strategies to specifically increase OE dimensions of accessibility, certainty, and importance.
A deeper understanding of how intervention components influence dimensions of OEs can inform the most effective ways to incorporate OEs in exercise interventions. Theoretical intervention fidelity refers to the consistency between intervention components that are theoretically hypothesized to produce change in theoretical constructs of interest (such as OEs) and the extent to which the components actually produce those changes.21 No studies have examined theoretical intervention fidelity of strategies intended to increase cancer survivors’ exercise OEs. Thus, the purpose of this manuscript is to report theoretical intervention fidelity and feasibility of delivering MOVING ON, an intervention to increase exercise outcome expectation accessibility, certainty, and importance, among breast cancer survivors.
1.1 |. Theoretical framework
The theoretical framework guiding this study is detailed elsewhere.22 This framework is based on evidence that exercise increases when breast cancer survivors (1) believe they can perform exercise (ie, have high exercise self-efficacy) and (2) expect desired outcomes will ensue10,18,19,23 (ie, have high exercise OEs).10,24 There are several dimensions of OEs including accessibility, certainty, and importance. This framework proposes that self-efficacy and all OEs increase exercise intentions (the most proximal predictor of behavior)14,25 and exercise.
2 |. METHODS
2.1 |. Design
This phase II feasibility study was a randomized 2-arm trial. This study was registered with Clinical.Trials.gov (NCT02348710) and received institutional Internal Review Board approval (Protocol #00059469).
2.2 |. Sample and setting
Participants were recruited in-person and through mailed invitations from a multidisciplinary breast cancer clinic at a tertiary cancer center. Eligibility criteria included (1) stage IA to IIB breast cancer diagnosis; (2) being 2 months to 10-year status postsurgery, radiation, and chemotherapy; (3) English speaking; (4) no evidence of recurrence, as determined by oncologic provider at a routine visit; (5) being inactive (self-reported ≤150 minute/week moderate-strenuous-intensity exercise) over the last month; (6) no contradictions to exercise based on the Physical Activity Readiness Questionnaire26; (7) access to a computer; and (8) possession of a smartphone. This phase II study was not powered for statistical testing. Rather, 60 participants were recruited to explore intervention effects, as part of assessing theoretical intervention fidelity,21 and feasibility of research methods.
2.3 |. Procedures
Consented participants were randomly assigned with equal probability to the intervention or attention control. A random number generator was used in excel to produce a randomization table. The randomization table was uploaded into Redcap, where participants were automatically randomized to group assignment. Demographic data were collected by medical chart review and interview. Objective exercise was collected by a Fitbit® that was synced to a Fitbit® account, created by a blinded research assistant, and mailed to participants. Participants who had a Fitbit® were allowed to use it and gave the study team direct access to data, by providing their username and password, to their established Fitbit® account. The Fitbit® was worn for 2 weeks, prior to receipt of intervention materials, to establish baseline exercise. Participants were mailed a MOVING ON intervention or attention control booklet,22 and were instructed to complete it within 1 week. A research assistant logged into Fitbit® accounts to retrieve objective exercise data at baseline, 4, 8, and 12 weeks postintervention. At these time points, subjective exercise, OEs, and selfefficacy were assessed through online surveys. Participants were allowed to keep the Fitbit as a thank you for study participation and compensation for their time.
2.4 |. Intervention
The intervention is described in detail elsewhere.22 Briefly, it consisted of a booklet containing narrative messages and writing and thinking activities to increase OE accessibility, certainty, and importance. The booklet provided a global overview of potential positive exercise outcomes for cancer survivors. The accessibility section instructed participants to list at least 3 strategies to help them think about outcomes they may experience if they exercise regularly. The certainty section contained 3 narrative messages (2 from breast cancer survivors who exercise regularly and 1 from an oncologist). Each survivor narrative included her photograph and summarized her personal story of (1) cancer treatments and side effects she experiences/ed and (2) outcomes obtained as a result of exercise and how achieving these outcomes helps her manage symptoms (eg, stress, pain). The oncologist’s narrative contained (1) her personal recommendation for breast cancer survivors to exercise and (2) outcomes she believes survivors may obtain, based upon current research. The importance section contained instructions to identify 3 most desired exercise outcomes and write about why each is personally important. The control arm received a similar booklet focused on diet only. The diet booklet included 1 oncologist and 1 survivor narrative, created by the research team. Both arms were also given the American Cancer Society’s diet and exercise recommendations for cancer survivors.
2.5 I. Measures
2.5.1 |. Fidelity
Theoretical intervention fidelity (ie, correspondence between intended and actual intervention effects on OEs)21 was measured by 9 questions (Table 1) and the direction of intervention effects. Fidelity was defined a priori as a score of greater than 2.0 for the 9 questions and OEs increasing more in the intervention compared to the control arm. Five open-ended questions (Table 1) were asked to provide insights into quantitative fidelity scores.
TABLE 1.
Feasibility questions, mean scores, and major themes from participants’ answers
| Quantitative Questions | n | Mean | SD | |
|---|---|---|---|---|
| How much did: | ||||
| 1. | The pamphlet make you think about how the benefits of exercise may apply to you as a cancer survivor? | 21 | 3.57 | 1.08 |
| 2. | The pamphlet make you think about why the benefits of exercise are personally important for you? | 22 | 3.68 | 1.17 |
| 3. | At least 1 of the survivor’s stories resemble your own experience with breast cancer treatment and side effects? | 22 | 2.50 | 1.14 |
| 4. | Both of the survivors’ stories resemble your own experience with breast cancer treatment and side effects? | 22 | 2.27 | 1.20 |
| 5. | At least 1 women’s’ stories make you feel that if you exercise you will experience benefits? | 21 | 3.43 | 1.33 |
| 6. | The survivor’s stories make you believe that you can exercise for at least 150 minutes per week at a moderate to strenuous intensity? | 22 | 3.45 | 1.18 |
| 7. | The oncologist’s story make you feel that if you exercise you will experience benefits? | 22 | 3.86 | 1.13 |
| 8. | The oncologist’s story make you believe that you can exercise for at least 150 minutes per week at a moderate to strenuous intensity? | 22 | 3.59 | 1.22 |
| 9. | The pamphlet increase how often you think about the reasons you want to exercise?/ | 22 | 2.95 | 1.13 |
| Average | 3.31 | 0.87 | ||
| Qualitative Questions | Common Themes | Example |
|---|---|---|
| 1. Please write the parts of the stories that you most related to or that you found most memorable. | Narrator’s exercise mode, frequency and/or duration (n = 6) Relating to cancer/treatment experience (n = 3) Shared emotions (n = 4) Inspiration (n = 5) |
“Carla became a walking machine and is fanatical about walking 15,000 steps a day.” “I was glad to read that one of the survivors had the same effect from tamoxifen.” “How scared having breast cancer makes you feel.” “You don’t realize that you have the power to make it until you hear someone else’s testimony. |
| 2. What did you do, if anything, that helped you think more often and remember your reasons to exercise? | Examples of strategies used (ie, meditation, email, calendar, phone reminder) (n = 4) Described current exercise (n = 10) | “Chose a sentence on exercise decreasing recurrence to email me and post on my google calendar.” “I started back walking my dog, exercising at the Senior Center.” |
| 3. What did you find most useful about the booklet? | Narrative stories (n = 13) Information (n = 6) | “That there were people like me with breast cancer and it made it feel like there was hope for me.” “There was a lot of good information that made me think about what I should be doing.” |
| 4. What did you find least useful about the booklet? | Easier experience than narrator (n = 2) Nothing (n = 5) | “It seemed like the survivors had a cancer more serious than mine. It was harder for me to relate to their stories.” “Nothing-it was really good information.” |
| Qualitative questions | Common themes | Example |
| 5. Please write any additional thoughts or comments you have about this booklet. | Should include nutrition more (n = 2). Positive feedback (n = 4) | “A bit more emphasis on what to eat. In my head that is more important than exercise.” “I thought the pamphlet was very well written and covered a great deal that a cancer survivor needs to know, and realize that other people share the same emotions!” |
Quantitative Likert scale rating 1 = not much, 2 = a little, 3 = somewhat, 4 = much, 5 = a great deal.
2.5.2 |. Outcome expectations
Outcome expectations were measured using a multidimensional exercise OE measure for breast cancer survivors.27 This measure assesses the dimensions of accessibility, certainty, and importance of 20 items that are possible outcomes of exercise, specific to breast cancer survivors, such as decreased recurrence risk. In a sample of 73 breast cancer survivors, the importance and accessibility measures demonstrated excellent reliability (Cronbach α = .96–.97) and stability over a 4-week time period (rs = 0.64–0.74).27
2.5.3 |. Exercise
Exercise intentions were measured with a 3-item scale that had excellent reliability (Cronbach α = .87) in a sample of colorectal cancer survivors.28 Exercise was measured subjectively as total weekly minutes of moderate and strenuous intensity exercise, using the Godin Leisure-Time Exercise Questionnaire (GLTEQ)29 and objectively using Fitbit®, which has demonstrated good reliability and validity for monitoring overground energy expenditure in lab-based treadmill and stair climber testing.30
2.5.4 |. Feasibility
Feasibility was measured by participant recruitment and retention and acquisition of Fitbit® data. Based on previous research, a priori feasibility criteria were set as recruitment of 60 participants in 6 months, total attrition less than 17%, and percent of Fitbit® data obtained being equal or greater than the percent of subjective exercise data obtained from the sample.22 Researcher notes were created to detail communication with participants about Fitbit® use, to explore reasons for data being obtained or not.
2.6 I. Analysis
2.6.1 |. Intervention Fidelity
Means and standard deviations were calculated for the quantitative fidelity questions. Common themes that inform the extent to which participants understood, completed, and found the intervention booklet useful were identified from qualitative responses to the open- ended fidelity questions.
2.6.2 |. Intervention effects
Statistically significant differences were noted between study arms for race, time since surgery, and time since chemotherapy (Table 2). All participants had a cancer-related surgery, but not chemotherapy. Thus, time since surgery and race were controlled for in all analyses. Between-wave missing data were accounted for through analyses with Proc Mixed in SAS version 9.4 (Cary, NC: SAS Institute Inc.). Indicated by Little’s (1988) missing completely at random (MCAR) test, within-wave missing data were missing completely at random MCAR (baseline χ2 = 744.590, P = 1.00; week 4 χ2 = 244.159, P = 1.000; week 8 χ2 = 412.123, P = 1.00; week 12 χ2 = 443.169, P = 1.00); thus, an expectation-maximization (EM) algorithm was used in SPSS for imputation.31
TABLE 2.
Participant demographics by intervention vs control group
| Intervention | Control | |
|---|---|---|
| Characteristic | n = 29 | n = 29 |
| X (SD) | X (SD) | |
| Age (years) | 59 (10) | 57 (12) |
| Months since cancer-related surgery* | 30 (24)* | 44 (29)* |
| Months since chemotherapy* n = 28 | 22 (12)* | 51 (26)* |
| Months since radiation n = 46 | 26 (25) | 40 (28) |
| Number of days Fitbit was not worn in the study (participants whose Fitbit data were accessible n = 35) | 26 (12) | 33 (16) |
| Height (inches) | 63.10 (2.45) | 63.83 (2.75) |
| Weight (pounds) | 175 (36) | 181 (43) |
| N (%) | N (%) | |
| Race* | ||
| African American or Black | 11 (19)* | 4 (7)* |
| White or Caucasian | 18 (31)* | 25 (43)* |
| Employment | ||
| Unemployed | 2 (6.8) | 0 (0) |
| Work part time | 4 (13.7) | 1 (3.4) |
| Work full time | 10 (34.4) | 14 (48.2) |
| Retired | 11 (37.9) | 12 (41.3) |
| Homemaker | 1 (3.4) | 1 (3.4) |
| Other | 1 (3.4) | 1 (3.4) |
| Marital status | ||
| Single, never married | 3 (10.3) | 3 (10.3) |
| Intervention | Control | |
| Characteristic | n = 29 | n = 29 |
| Married or domestic partnership | 17 (58.6) | 23 (79.3) |
| Widowed | 3 (10.3) | 1 (3.4) |
| Divorced/separated | 6 (20.5) | 2 (6.8) |
| Health insurance | 28 (97) | 29 (100) |
| Cancer stage | ||
| Ia | 14 (48.2) | 11 (37.9) |
| Ib | 1 (3.4) | 2 (6.8) |
| IIa | 10 (34.4) | 11 (37.9) |
| IIb | 4 (13.7) | 5 (17.2) |
| Surgery type | ||
| Mastectomy | 10 (34.4) | 9 (31) |
| Partial mastectomy | 0(0) | 4 (13.7) |
| Lumpectomy | 21 (72.4) | 16 (55.1) |
| Taking aromatase inhibitors | ||
| Arimidex (anastrozole) | 8 (27.5) | 4 (13.7) |
| Aromasin (exemestane) | 1 (3.4) | 1 (3.4) |
| Femera (letrozole) | 3 (10.3) | 5 (17.2) |
| Taking selective estrogen receptor modulator | 6 (20.6) | 13 (44.8) |
| Wore Fitbit prior to study | 6 (20.6) | 12 (41.3) |
| Able to obtain Fitbit data | 17 (58.62) | 18 (41.38) |
P < .05.
Two-level modeling was done using Proc Mixed. Assumptions of mixed models were tested. Outcomes were modeled as a linear function of time to create growth trends of the trajectory of change over 12 weeks, that were modeled as a linear function of the study arm. Models containing week, arm, their interaction, significant covariates, and interactions were built for each outcome. Nonsignificant items were removed until a final parsimonious model was achieved. The level of significance was set at 0.05, 2-tailed. Effect sizes were calculated by dividing each beta coefficient by the residual error variance for each outcome.
2.6.3 |. Feasibility of research methods
Descriptive statistics were conducted to assess participant recruitment and retention at each time point and days Fitbit® data were obtained.
3 I. RESULTS
3.1 I. Sample characteristics
The sample consisted of 60 breast cancer survivors, 74% Caucasian, 26% African American, with mean age 58 years, and mean time since diagnosis of 3 years. Participant demographics and medical characteristics are detailed in Table 2.
3.2 |. Intervention fidelity
Twenty-two intervention participants completed the 4-week post intervention fidelity measures. They reported completing 3 quarters of the intervention booklet. As detailed in Table 1, across the 9 quantitative fidelity questions, the mean score is 3.31 (SD = 0.87), which corresponds with “somewhat” on the Likert scale ratings. All items individually achieved an a priori feasibility score of greater than 2.
Responses to qualitative questions revealed positive general feedback and that most participants thought everything in the booklet was useful. The narrative stories were reported as the most useful section. The section that asked participants to develop strategies to think about the reasons they want to exercise appeared to be the least effective section. Examples of participant answers and major themes or common responses to all qualitative questions are detailed in Table 1.
3.3 |. Intervention effects
Across all time points, all measures demonstrated good to excellent reliability (OEs: Cronbach α = .95, intentions: Cronbach α = .86, and self-efficacy: Cronbach α = .86). The final models are detailed in Table 3. Overall OEs, and accessibility, certainty, and importance of all dimensions and exercise intentions increased a nonsignificant 0.01 point every 4 weeks in the intervention arm compared to the control arm (P = .3555, .6578, .5026, and .6254, respectively). Subjective exercise (weekly minutes) increased 2 minutes, and objective exercise increased by 970 steps, every 4 weeks in the intervention arm compared to the control arm (P = .2676 and .0283, respectively). All effect sizes were small ranging from 0.01 to 0.09. Race stood out as a significant independent predictor of OEs, at all time points as scores range from 0.3 to 0.7 points higher (P < .05) for African American (AA) compared to Caucasian participants. Time since treatment had no significant effects on OEs or exercise.
TABLE 3.
Final models of intervention effects
| Outcome Variable |
Arm | Week | Race 1 = Black 2 = White |
Self- Efficacy |
Time Since Treatment |
Arm × Week Interaction |
Week × Self‐Efficacy Interaction |
|
|---|---|---|---|---|---|---|---|---|
| Overall OEs | ||||||||
| Estimate | 0.1803 | −0.0044 | −0.3857* | 0.2047* | −0.0050* | 0.0074 | ||
| Standard | ||||||||
| Error | 0.0946 | 0.0057 | 0.1047 | 0.0365 | 0.0017 | 0.0080 | ||
| Effect size | 0.66 | −0.02 | −1.42 | 0.75 | −0.02 | 0.03 | ||
| OE accessibility | ||||||||
| Estimate | 0.4651* | −0.0072 | −0.6720* | 0.4776* | −0.0098* | 0.0058 | ||
| Standard | ||||||||
| Error | 0.1345 | 0.0093 | 0.1511 | 0.0576 | 0.0025 | 0.0131 | ||
| Effect size | 1.05 | −0.02 | −1.52 | 1.08 | −0.02 | 0.01 | ||
| OE certainty | ||||||||
| Estimate | −0.0116 | 0.0075 | −0.3474* | 0.0073 | ||||
| Standard | ||||||||
| Error | 0.1114 | 0.0076 | 0.0977 | 0.0108 | ||||
| Effect size | −0.04 | 0.02 | −1.15 | 0.02 | ||||
| OE importance | ||||||||
| Estimate | 0.0669 | −0.0972* | −0.3558* | −0.0900 | 0.0092 | 0.0292* | ||
| Standard | ||||||||
| Error | 0.1165 | 0.0220 | 0.1033 | 0.06441 | 0.0097 | 0.0075 | ||
| Effect size | 0.20 | −0.29 | −1.08 | −0.27 | 0.09 | 0.09 | ||
| Exercise intentions | ||||||||
| Estimate | 0.0102 | −0.0196 | 0.9721* | 0.0092 | ||||
| Standard | ||||||||
| Error | 0.1816 | 0.0133 | 0.0771 | 0.0188 | ||||
| Effect size | 0.02 | −0.03 | 1.52 | 0.01 | ||||
| Weekly minutes | ||||||||
| Estimate | −15.9501 | −10.4679* | 15.6968 | 2.0404 | 4.5383* | |||
| Standard | ||||||||
| Error | 18.1657 | 4.2042 | 11.6075 | 1.8317 | 1.4233 | |||
| Effect size | −0.25 | −0.17 | 0.25 | 0.03 | 0.07 | |||
| Steps | ||||||||
| Estimate | −6308.11 | −204.69 | 8721.46* | 969.71* | ||||
| Standard | ||||||||
| Error | 4153.19 | 310.20 | 1827.16 | 436.60 | ||||
| Effect size | −0.42 | −0.01 | 0.59 | 0.07 | ||||
| Exercise self‐efficacy | ||||||||
| Estimate | −0.0338 | 0.0166 | −0.0014 | |||||
| Standard | ||||||||
| Error | 0.1639 | 0.0108 | 0.0152 | |||||
| Effect size | −0.07 | 0.03 | −0.00 |
No value indicates the variable was not part of the final model.
P < .05.
3.4 |. Feasibility
3.4.1 |. Recruitment and retention
Target enrollment (n = 60) was achieved in 7 weeks, over which time the researcher spent 252 hours (roughly 3 hours per day, 3 days per week) in the clinic recruiting participants. One participant from each arm withdrew prior to baseline data completion. One participant did not like wearing the Fitbit®, and it is unknown why the other with-drew. Data completion rates at baseline, 4, 8, and 12 weeks post intervention were 78%, 74%, 74%, and 66%, respectively. Overall attrition was 34%. Study flow is detailed in Figure 1. A priori recruitment feasibility criteria (60 participants in 6 months) were met, but retention (total attrition less than 17%) was not.
FIGURE 1.
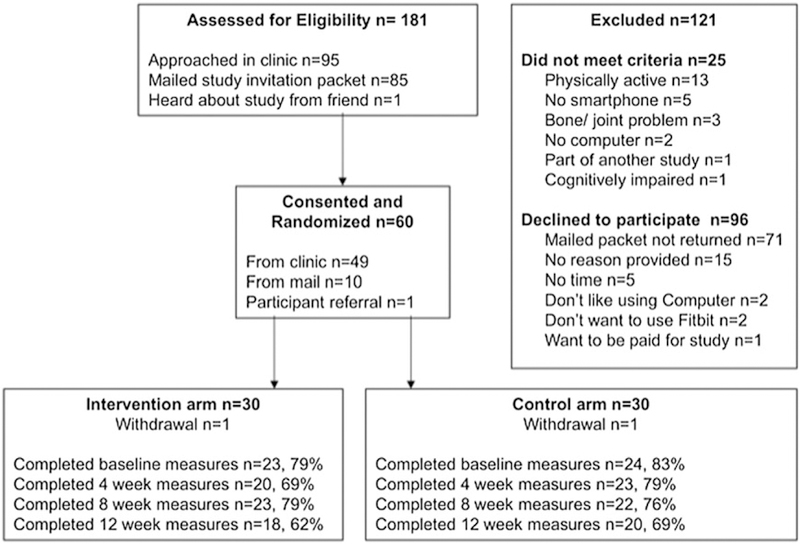
Study flow
3.4.2 |. Fitbit
Twelve participants used their own Fitbit®, of which 11 provided access to Fitbit data. Fifteen participants synced the Fitbit® to their smartphone with no reported problems or assistance. Two participants reported requiring help from a spouse, and 7 contacted the researcher for help with initially syncing the Fitbit® to their smartphone. Throughout the study period, participants contacted the researcher 15 times regarding the Fitbit®. Reasons included questions about syncing (n = 7), settings (n = 4), low battery (n = 4), loving it (n = 3), and not liking to wear it (n = 2). No Fitbit® data were obtained for 23 participants with study-issued Fitbits because it was never synced (n = 12) or because login information did not permit access to the Fitbit® account (n = 11), indicating either the participant changed the study issue password or a researcher error in documentation.
Ultimately, Fitbit® data were obtained for 60% of participants compared to 73% of participants who provided self-report exercise data. Among participants whose data were accessed, they wore the Fitbit for 71% of the 105 study days (mean = 75 days, SD = 30). Fitbit as an objective measure did not meet a priori feasibility criteria of equal to or greater than the subjective exercise measure. There were no significant baseline measured differences between participants whose Fitbit® data were and were not obtained.
4 |. CONCLUSIONS
Intervention fidelity achieved a priori criteria. Overall, the intervention components impacted OE dimensions as intended. Outcome expectations and exercise increased more in the intervention compared to control arm, indicating that while not powered to detect significance, the intervention produced desired effects. Further, self-efficacy did not change throughout the intervention period indicating that the intervention solely targeted OEs as intended. The narratives appeared to be the most effective part of the intervention. When a person identifies with a narrator, she believes that because they are similar, she may have a similar experience.32,33 Future research may make narrative messages even more powerful by tailoring to individuals based on their demographic characteristics, cancer treatment, and side effects experienced. The least useful part of the intervention was the section primarily targeting OE accessibility, where participants were instructed to list 3 things they will do to think more often about the reasons they exercise. Most participants listed the exercise plans in this section. It is possible that this section was not effective because participants did not understand the instructions, or that people want more support to plan how they will keep their exercise goals mentally accessible. Future research is needed to understand how to increase OE accessibility.
Target enrollment was achieved in 17 weeks. This compares favorably to similar studies which required 12 months to recruit 40 participants at clinic follow-up visits 34 and 23 months to recruit 210 participants through the mail.35 High recruitment rates may be because of a referring nurse practitioner being on the study team and screening patients and the primary investigator spending considerable time (about 250 hours) in the clinic.
The present study had a high attrition rate (34%) compared to other home-based exercise intervention for breast cancer survivors in which attrition ranged from 13% to 20%.36,37 Response rate may be improved in future research by showing participants how to access online measures at the time of enrollment, providing an option for paper and pen measures or be increasing researcher and participant interaction.
Fitbit® data were obtained for 60% of participants for an average 71% of study days. This is similar to the amount of subjective exercise data obtained through online surveys in this study, as online measures were completed by 73% of participants. Several strategies should be used in future research to improve data acquisition through Fitbit®. Specifically, a researcher should sync the Fitbits to the participants’ smartphones, login information should be double documented, and Fitbit accounts should be accessed by the research team at several time points during the study, to confirm data capture and trouble shoot as needed.
An important incidental finding is that AA participants had higher OEs at all time points compared to Caucasian participants. This is consistent with other research that indicates Black breast cancer survivors report more expected exercise benefits compared to Whites.38,39 Thus, increasing OEs may not be the most effective means to increase exercise among AA cancer survivors, and it is critical to further explore and understand racial and cultural differences in exercise when designing future interventions.
4.1 |. Study limitations
Attrition of 37% and not having Fitbit® data for 40% of participants may have caused bias in the results. Additionally, high mean OE scores (3.2–4.4, on a 1–5 Likert scale) at baseline indicate possible ceiling effects. Thus, the OE measure may not have been sensitive enough to note significant increases in OEs. Finally, selection bias may have impacted study results because people who have positive attitudes toward exercise may be more likely to enroll in a healthy lifestyle intervention study. These people may have greater motivation to exercise and be more sensitive to the intervention.
4.2 |. Clinical implications
Findings indicate that the most effective part of the intervention was the narrative stories. Providers may motivate increased exercise among patients by providing simple print brochures with stories about survivors who successfully manage late and long-term effects with regular exercise. This approach is low-cost and simple to implement in a busy clinic setting. Findings related to feasibility of research methods provide insights into improving study attrition and data collection for a larger future trial, powered to test intervention effect sizes. Finally, information about how to best obtain Fitbit data as an objective exercise measure in research is revealed from study findings. Based on study findings, it is critical to assist participants with syncing the Fitbit to their smartphone and to confirm data acquisition at several time points during the study.
Acknowledgments
Funding information
National Institute of Nursing Research, Grant/Award Numbers: 1F31NR015690–0 and 2T32NR007091; Oncology Nursing Society Foundation
REFERENCES
- 1.O’Neill SC, DeFrank JT, Vegella P, et al. Engaging in health behaviors to lower risk for breast cancer recurrence. PLOS ONE. 2013;8(1):e53607. 10.1371/journal.pone.0053607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loprinzi PD, Lee H. Rationale for promoting physical activity among cancer survivors: literature review and epidemiologic examination. Oncol Nurs Forum. 2014;41(2):117–125. 10.1188/14.ONF.117-125 [DOI] [PubMed] [Google Scholar]
- 3.Smith SG, Chagpar AB. Adherence to physical activity guidelines in breast cancer survivors. Am Surg. 2010;76(9):962–965. [PubMed] [Google Scholar]
- 4.Blanchard CM, Courneya KS, Stein K. Cancer survivors adherence to lifestyle behavior recommendations and associations with health- related quality of life: results from the American Cancer Society’s SCS-II. J Clin Oncol. 2008;26(13):2198–2204. 10.1200/JCO.2007.14.6217 [DOI] [PubMed] [Google Scholar]
- 5.Rock CL, Doyle C, Demark-Wahnefried W, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012;62(4):243–274. 10.3322/caac.21142 [DOI] [PubMed] [Google Scholar]
- 6.Schmitz KH, Courneya KS, Matthews C, et al. American College of Sports Medicine roundtable on exercise guidelines for Cancer survivors. Med SciSports Exerc. 2010;42(7):1409–1426. 10.1249/MSS.0b013e3181e0c112 [DOI] [PubMed] [Google Scholar]
- 7.Bock C, Schmidt ME, Vrieling A, Chang-Claude J, Steindorf K. Walking, bicycling, and sports in postmenopausal breast cancer survivors-results from a German patient cohort study. Psychooncology. 2013;22(6):1291–1298. 10.1002/pon.3134 [DOI] [PubMed] [Google Scholar]
- 8.Pinto BM, Trunzo JJ, Reiss P, Shiu S-Y. Exercise participation after diagnosis of breast cancer: trends and effects on mood and quality of life. Psychooncology. 2002;11(5):389–400. 10.1002/pon.594 [DOI] [PubMed] [Google Scholar]
- 9.Hirschey R, Docherty SL, Pan W, Lipkus I. Exploration of exercise outcome expectations among breast cancer survivors. Cancer Nurs. February 2016;1(2):E39–E46 10.1097/NCC.0000000000000362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bandura A Health promotion by social cognitive means. Health Educ Behav. 2004;31(2):143–164. 10.1177/1090198104263660 [DOI] [PubMed] [Google Scholar]
- 11.Olson JM, Roese NJ, Zanna MP. Expectancies In: Higgins ET, Kruglanski AW, eds. Social Psychology: Handbook of Basic Principles. New York, NY: Guilford Press; 1996:211–238. [Google Scholar]
- 12.Petty RE, Krosnick JA. In: Petty RE, Krosnick JA, eds. Attitude Strength. Psychology Press; 2014. [Google Scholar]
- 13.Gross SR, Holtz R, Miller N. Attitude certainty In: Petty RE, Krosnick JA, eds. Attitude Strength: Antecedents and Consequences. New Jersey: Lawrence Erlbaum; 1995:215–246. [Google Scholar]
- 14.Ajzen I The theory of planned behavior. Organ Behav Hum Decis Process. 1991;50(2):179–211. [Google Scholar]
- 15.Prochaska JO, Redding CA, Evers KE. The transtheoretical model and stages of change In: Glanz K, Rimer BK, Lewis FM, eds. Health Behavior and Health Education. 3rd ed San Francisco: Jossey-Bass; 2002:99–120. [Google Scholar]
- 16.Brassington GS, Atienza AA, Perczek RE, DiLorenzo TM, King AC. Intervention-related cognitive versus social mediators of exercise adherence in the elderly. Am J Prev Med. 2002;23(2 Suppl):80–86. [DOI] [PubMed] [Google Scholar]
- 17.Schutzer KA, Graves BS. Barriers and motivations to exercise in older adults. Prev Med. 2004;39(5):1056–1061. 10.1016/j.ypmed.2004.04.003 [DOI] [PubMed] [Google Scholar]
- 18.Rogers LQ, Hopkins-Price P, Vicari S, et al. A randomized trial to increase physical activity in breast cancer survivors. Med Sci Sports Exerc. 2009;41(4):935–946. 10.1249/MSS.0b013e31818e0e1b [DOI] [PubMed] [Google Scholar]
- 19.Hatchett A, Hallam JS, Ford MA. Evaluation of a social cognitive theory-based email intervention designed to influence the physical activity of survivors of breast cancer. Psychooncology. 2013;22(4):829–836. 10.1002/pon.3082 [DOI] [PubMed] [Google Scholar]
- 20.Short CE, James EL, Girgis A, D’Souza MI, Plotnikoff RC. Main outcomes of the move more for life trial: a randomised controlled trial examining the effects of tailored-print and targeted-print materials for promoting physical activity among post-treatment breast cancer survivors. Psychooncology. 2014;24(7):771–778. 10.1002/pon.3639 [DOI] [PubMed] [Google Scholar]
- 21.Ibrahim S, Sidani S. Intervention fidelity in interventions: an integrative literature review. Res Theory Nurs Prac. 2016;30(3):258–271. 10.1891/1541-6577.30.3.258 [DOI] [PubMed] [Google Scholar]
- 22.Hirschey R, Kimmick G, Hockenberry M, Shaw R, Pan W, Lipkus I. Protocol for Moving On: a randomized controlled trial to increase outcome expectations and exercise among breast cancer survivors. Nursing Open. January 2018:1–8. 10.1002/nop2.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rogers LQ, Fogleman A, Trammell R, et al. Effects of a physical activity behavior change intervention on inflammation and related health outcomes in breast cancer survivors: pilot randomized trial. Integr Cancer Ther. 2013;12(4):323–335. 10.1177/1534735412449687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loprinzi PD, Cardinal BJ. Self-efficacy mediates the relationship between behavioral processes of change and physical activity in older breast cancer survivors. Breast Cancer. 2013;20(1):47–52. 10.1007/s12282-011-0298-x [DOI] [PubMed] [Google Scholar]
- 25.Scholz U, Keller R, Perren S. Predicting behavioral intentions and physical exercise: a test of the health action process approach at the intrapersonal level. Health Psychol. 2009;28(6):702–708. [DOI] [PubMed] [Google Scholar]
- 26.Thomas S, Reading J, Shephard RJ. Revision of the physical-activity readiness questionnaire (par-Q). Can J Sport Sci. 1992;17(4):338–345. [PubMed] [Google Scholar]
- 27.Hirschey R, Pan W, Hockenberry M, Kimmick G, Shaw R, Lipkus I. What do breast cancer survivors expect from exercise? Cancer Nursing. 2018. 10.1097/NCC.0000000000000631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hirschey R, Lipkus I, Jones L, Demark-Wahnefried W, Sloane R. Do sedentary colorectal cancer survivors view lack of exercise as a risk factor for cancer recurrence? Oncol Nurs Forum Online Exclusive Abstracts. 10.1188/13.ONF.E382-E430 [DOI] [Google Scholar]
- 29.Godin G The Godin-Shephard leisure-time physical activity questionnaire. Health Fit J Can. 2011;4(1):18–22. [Google Scholar]
- 30.Adam Noah J, Spierer DK, Gu J, Bronner S. Comparison of steps and energy expenditure assessment in adults of Fitbit tracker and ultra to the Actical and indirect calorimetry. J Med Eng Technol. 2013;37(7):456–462. 10.3109/03091902.2013.831135 [DOI] [PubMed] [Google Scholar]
- 31.Do CB, Batzoglou S. What is the expectation maximization algorithm? Nat Biotechnol. 2008;26(8):897–899. 10.1038/nbt1406 [DOI] [PubMed] [Google Scholar]
- 32.Hinyard LJ, Kreuter MW. Using narrative communication as a tool for health behavior change: a conceptual, theoretical, and empirical overview. Health Educ Behav. 2007;34(5):777–792. 10.1177/1090198106291963 [DOI] [PubMed] [Google Scholar]
- 33.Kreuter MW, Buskirk TD, Holmes K, et al. What makes cancer survivor stories work? An empirical study among African American women. J Cancer Surviv. 2008;2(1):33–44. 10.1007/s11764-007-0041-y [DOI] [PubMed] [Google Scholar]
- 34.Fields J, Richardson A, Hopkinson J, Fenlon D. Nordic walking as an exercise intervention to reduce pain in women with aromatase inhibitor-associated arthralgia: a feasibility study. J Pain Symptom Manage. 2016;52(4):548–559. 10.1016/j.jpainsymman.2016.03.010 [DOI] [PubMed] [Google Scholar]
- 35.Befort CA, Klemp JR, Fabian C, et al. Protocol and recruitment results from a randomized controlled trial comparing group phone-based versus newsletter interventions for weight loss maintenance among rural breast cancer survivors. Contemp Clin Trials. 2014;37(2):261–271. 10.1016/j.cct.2014.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lahart IM, Metsios GS, Nevill AM, Kitas GD, Carmichael AR. Randomised controlled trial of a home-based physical activity intervention in breast cancer survivors. BMC Cancer. 2016;16(1):234 10.1186/s12885-016-2258-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pinto BM, Rabin C, Abdow S, Papandonatos GD. A pilot study on disseminating physical activity promotion among cancer survivors: a brief report. Psychooncology. 2008;17(5):517–521. 10.1002/pon.1268 [DOI] [PubMed] [Google Scholar]
- 38.Spector D, Battaglini C, Groff D. Perceived exercise barriers and facilitators among ethnically diverse breast cancer survivors. Oncol Nurs Forum. 2013;40(5):472–480. 10.1188/13.ONF.472-480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dean LT, Brown J, Coursey M, Schmitz KH. Great expectations: racial differences in outcome expectations for a weight lifting intervention among black and white breast cancer survivors with or without lymphedema. Psychooncology. 2016;25(9):1064–1070. 10.1002/pon.4175 [DOI] [PMC free article] [PubMed] [Google Scholar]


