Figure 2. Monomeric Eg5 promotes microtubule assembly and enhances microtubule stability.
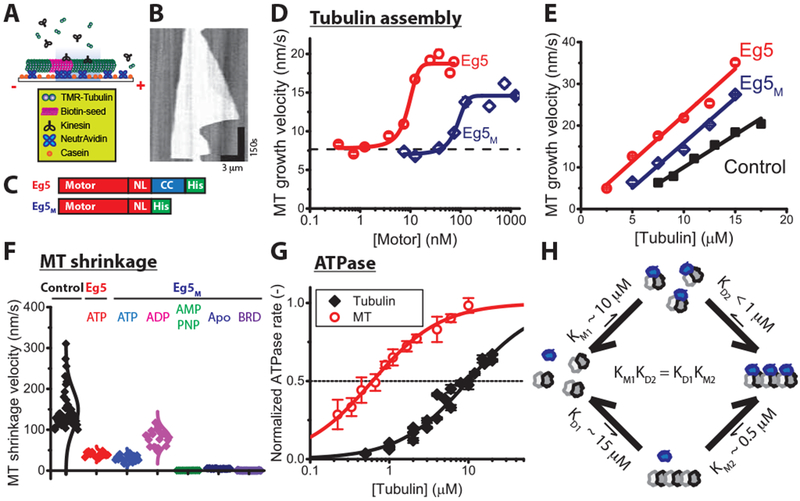
(A-E) Microtubule growth rates. (A) Schematic of microtubule dynamics assays, in which free tubulin is grown off of biotinylated GMPCPP microtubule seeds. (B) Example kymograph showing unlabeled seed and growth from both ends over time, visualized by Interference Reflection Microscopy. (C) Diagrams of constructs. Dimeric Eg5 was made by replacing the native Eg5 coiled-coil with the kinesin-1 coiled-coil to ensure stable dimerization [21, 45]. (D) Motor-dependent microtubule growth velocities at 7.5 μ M tubulin and varying motor concentrations at 25 °C (mean ± SEM; nMT = 47-291 for Eg5 and 11-147 for Eg5M). (E) Tubulin-dependent microtubule elongation rates at saturating motor concentrations (61 nM Eg5 and 1.25 μ M Eg5M; mean ± SEM; nMT = 53-114, 62-142, and 37-427 for control, Eg5 and Eg5M, respectively). (F) Effects of Eg5 on microtubule shrinkage rates following tubulin washout at 22 °C, visualized by TIRF microscopy. Dimeric Eg5 was used at 80 nM and monomeric Eg5M was used at 10 μ M under different nucleotide conditions (nMT = 12-42). (G) Tubulin- and microtubule-stimulated ATPase of monomeric Eg5 at 22 °C. ATPase rates are normalized to maximal for each substrate. Based on the KM values, the apparent affinity of Eg5M for tubulin (KM1 = 9.9 ± 1.2 μM) is 20-fold lower than its affinity for microtubules (KM2 = 0.6 ± 0.03 μM), which suggests that Eg5 motors favor the straight conformation of tubulin (mean ± SEM; nMT = 5; nTub = 3). Mean ± SEM. (H) Schematic of affinity-driven polymer assembly. The twenty-fold difference of Eg5 affinity in Figure 2G generates a potential to facilitate tubulin assembly, consistent with the differential critical concentrations of microtubule growth in the presence and absence of monomeric Eg5 in Figure 2E. See also Figure S2.
