Figure 4. Loop11-helix 4 mediates Eg5 end-accumulation and microtubule polymerase activity.
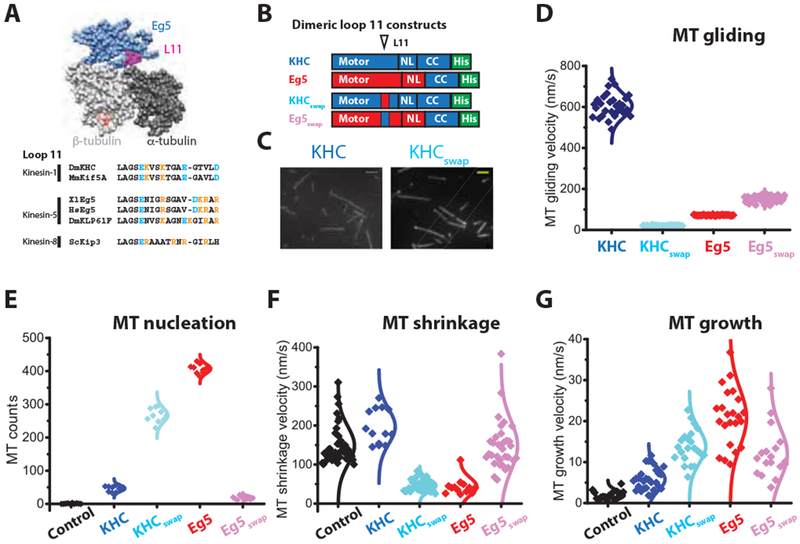
(A) Structure of tubulin-bound Eg5, highlighting the location of L11-α4 at the α/β-tubulin interface (PDB: 4AQW). Comparison of L11-α4 sequences, highlighting positions of basic residues. Full alignments are shown in Figure S4A. (B) Diagram of L11-α4-swapped mutants, KHCswap and Eg5Swap. (C) Swapping Eg5 L11-α4 into KHC is sufficient to confer end-binding activity. Scale bar: 2 μ m. (D) L11-α4 strongly affects microtubule gliding speeds, consistent with it regulating the strong-binding state of the motor. Velocities: KFIC, 606 ± 56 nm/s; KFICswap: 21 ± 2 nm/s; Eg5: 72 ± 2 nm/s; Eg5swap: 153 ± 16 nm/s; nMT = 26-37. Mean ± SEM. (E) Number of newly formed microtubule across motor species. 5 μ M TMR-labeled microtubules plus 10 vol% DMSO were assembled in the presence or absence of 130 nM motors at 37 °C. Representative images are shown in Figure S4B. (F) Microtubule shrinkage rates following tubulin-washout, showing that L11-α4 confers microtubule stabilization activity. Assays used 80 nM dimeric motors; control and Eg5 groups were taken from Figure 2F (nMT = 13-42). (G) Microtubule growth rates showing that L11-α4 contributes to growth enhancement activity of Eg5. Assays used 20 μM tubulin in the presence or absence of 10 nM dimeric motors; control and Eg5 groups were taken from Figure 2D (nMT = 16-27). See also Figure S4.
