| Drug name (generic) | Bosutinib monohydrate (BOSULIF®) |
|---|---|
| Phase (for indication under discussion) | Bosutinib has been studied for its safety and efficacy in patients with Philadelphia chromosome positive (Ph+) leukemia with resistance or intolerance to prior tyrosine kinase inhibitor therapy. |
| Indication (specific to discussion) | Bosutinib is currentlyFDA approved and indicated for the treatment of chronic, accelerated, or blast phase Ph+ chronic myeloid leukemia in adult patients with resistance or intolerance to prior therapy. |
| Pharmacology description/mechanism of action | Dual Abl/Src tyrosine kinase inhibitor |
| Route of administration | BOSULIF ® tablets are orally administered, available in two strengths, 100mg or 500mg tablets. |
| Chemical structure | C26H29Cl2N5O3 ∙ H2O (monohydrate)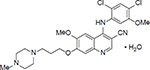 (Image taken from rxlist.com) |
| Pivotal trial(s) | Study 200, BELA Trial 32 |

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.