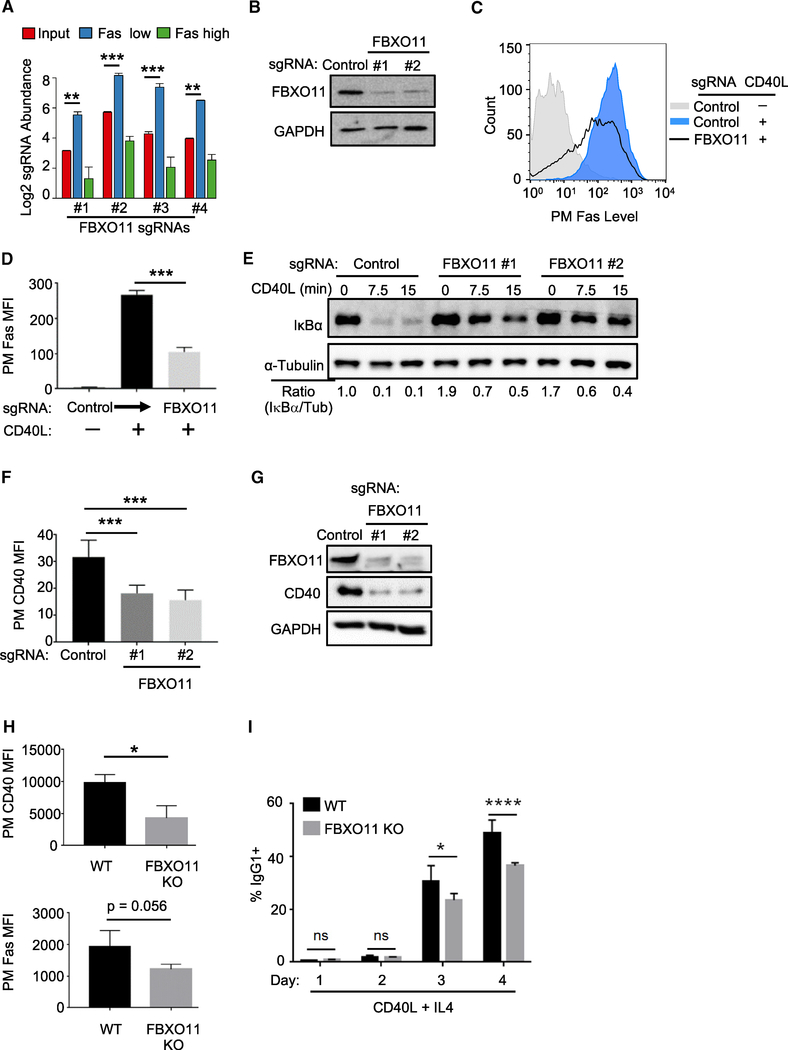
Figure 2. FBXO11 Is a Critical Dependency Factor for B Cell CD40 Expression.
(A) Log2-normlized FBXO11 sgRNA abundances within the indicated CRISPR screen cell populations. CRISPR Daudi cell library (red), in the sorted Faslow (blue) and Fashigh (green) populations are shown. Mean ± SD of two input libraries and four screen replicates are shown.
(B) Immunoblot analysis of whole cell extracts (WCE) from Cas9+ Daudi B cells expressing the indicated control or independent FBXO11-targeting sgRNAs.
(C) FACS analysis of PM Fas levels in Cas9+ Daudi B cells expressing the indicated control or FBXO11 targeting sgRNA and stimulated by 50 ng/mL Mega-CD40L for 48 h as shown.
(D) FACS analysis of PM Fas median fluorescence intensity (MFI) as in (B) from n = 3 replicates.
(E) CD40 canonical NF-κB pathway IκBα and tubulin load control levels in WCE from control or FBXO11 sgRNA expressing Daudi B cells treated with 50 ng/mL Mega-CD40L for the indicated times. The ratios of IκBα to tubulin (tub) abundances are shown beneath.
(F) FACS analysis of PM CD40 MFI in Daudi B cells expressing the indicated sgRNA and stimulated by Mega-CD40L 50 ng/mL for 48 h, as indicated, using n = 3 replicates.
(G) Immunoblot analysis of WCE from Cas9+ Daudi B cells expressing the indicated control or FBOX11 sgRNA from a replicate shown in (F).
(H) FACS analysis of PM CD40 and Fas levels in primary spleen B cells from n = 3 wild type (WT) or FBXO11 KO mice stimulated by anti-CD40 agonist antibody (1 μg/mL) and recombinant mouse IL-4 (20 ng/mL) for 48 h.
(I) Percentages of IgG1 + B cells obtained from n = 5 WT or FBXO11 KO mice stimulated for the indicated days with anti-CD40 antibody (1 μg/mL) and recombinant mouse IL-4 (20 ng/mL).
All immunoblots were representative of at least n = 3 replicates. Mean + SD are shown in (D), (F), (H), and (I). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Cas9+ Daudi B cells were used for (A)–(G).
See also Figures S2 and S3.