Abstract
Background:
Recent work has implicated disinhibited eating behaviors (DEB) as a potential pathway toward obesity development in children. However, the underlying neurobiology of disinhibited eating behaviors in young, healthy weight children, prior to obesity development, remains unknown.
Objectives:
This study tested the relationship between DEB and intrinsic neuronal activity and connectivity in young children without obesity.
Methods:
Brain networks implicated in overeating including reward, salience and executive control networks, and the default mode network were investigated. DEB was measured by the Eating in the Absence of Hunger (EAH) paradigm with post-lunch kilocalories consumed from highly palatable foods (EAH kcal) used as the predictor. Intrinsic neuronal activity within and connectivity between specified networks was measured via resting state functional magnetic resonance imaging. Eighteen typically developing children (mean age = 5.8 years) were included. Results: EAH kcal was positively associated with activity of the nucleus accumbens, a major reward network hub (p < 0.05, corrected). EAH kcal was negatively associated with intrinsic prefrontal cortex connectivity to the striatum (p < 0.01, corrected).
Conclusions:
These results suggest that neural aspects of DEB are detectable in young children without obesity, providing a potential tool to better understand the development of obesity in this population.
Keywords: eating in the absence of hunger, obesity, pediatric, functional magnetic resonance imaging, brain networks
INTRODUCTION
Recent evidence suggests that overweight and obesity prevalence in youth 2 – 19 years-old is steadily increasing in the United States. Furthermore, severe obesity (Class I obesity: body mass index >120% of the 95th percentile) among young children 2 – 5 years old has risen sharply 1. Children with obesity are five times more likely to struggle with obesity as adults 2, highlighting childhood as a window of opportunity to intervene and reduce the burden of obesity in adulthood. Although development of obesity is a multifaceted process, consumption of energy beyond daily caloric needs is one clear etiologic driver of weight gain, indicating the importance of eating behavior in obesity etiology.
Disinhibited eating behaviors, or episodes when food is consumed for reasons other than hunger, promote overeating in children and are associated with longitudinal weight gain and obesity prevalence in pediatric groups 3–12. Hedonically-motivated disinhibited eating behavior, such as eating in the absence of hunger, is of particular concern given the ubiquitous availability of highly palatable, hedonic foods in modern society 13. Eating in the absence of hunger is characterized by consumption of highly palatable foods beyond satiation 7. Conceptually, eating in the absence of hunger is based on Schachter’s externality theory of obesity 14 which posits that overeating is the result of environmental and food-related stimuli (e.g. taste, smell, sight) which result in the overriding of an individual’s satiety responsiveness.
Consistent with the externality theory of obesity, a growing body of literature has shown differential sensory processing in the brain for hedonic taste and visual stimuli in youth with and without obesity 15–19. Specifically, brain regions sub-serving sensory processing and salience valuation of stimuli such as the insula 20–22, and reward circuitry including the ventral striatum, amygdala, and ventral tegmental area 23,24, are altered in hedonically-motivated disinhibited eating behaviors 19,25,26. Additionally, functional dysregulation of brain regions involved with cognitive and attentional control processes, specifically inhibitory control, are also implicated in hedonically-motivated disinhibited eating behaviors. These regions include the frontal poles and the superior and inferior frontal gyri 18,25,27. Together, these data suggest that hedonically-motivated disinhibited eating behavior may be a manifestation of disrupted response to food cues within these brain regions. Furthermore, these brain regions participate in large functional networks, specifically the salience (e.g. insula), reward (e.g. basal ganglia: ventral striatum/nucleus accumbens), executive control (e.g. frontal poles), and default mode (e.g. posterior prefrontal and medial prefrontal cortices) networks 28. Disruption in the communication or connectivity between these networks could also contribute to hedonically-motivated disinhibited eating behavior.
To date, however, very little is known about the developmental trajectory of hedonically-motivated disinhibited eating behavior and its underlying neurobiology prior to obesity development. This gap in the scientific literature is further exacerbated by the lack of observational eating behavior measures that quantify actual overeating. While significant work has examined the brain’s response to taste and visual stimuli in individuals with obesity 29–34, no study to date has investigated the relationship between laboratory-observed, hedonically-motivated eating behavior and intrinsic neuronal activity and connectivity in young children without obesity.
In this study, intrinsic within-network neuronal activity and between-network connectivity were investigated via resting state functional magnetic resonance imaging. Hedonically-motivated disinhibited eating behavior was measured via the Eating in the Absence of Hunger test paradigm in a group of non-obese, typically developing 4 to 6-year-old children in the state of Colorado in the United States. Given prior observations suggesting that salience, reward, and default mode network regions are hyperactive in response to food stimuli (e.g. visual, taste) and executive control network regions are hypoactive, it was hypothesized that greater eating in the absence of hunger would be positively associated with neuronal activity within the salience, basal ganglia (reward), and default mode networks, and lower neuronal activity within the executive control network. Furthermore, based on the functional roles of the brain regions within each network of interest and their contribution to promoting or limiting eating behaviors, it was hypothesized that connectivity between the networks would be significantly associated with eating in the absence of hunger. Specifically, it was hypothesized that the salience and basal ganglia networks would be positively associated with eating in the absence of hunger. It was also hypothesized that connectivity between the salience and executive control networks, and basal ganglia and executive control networks would be negatively associated with eating in the absence of hunger.
METHODS
Participants
Data used in this study were obtained from participants enrolled in the ongoing Healthy Start study, a pre-birth longitudinal cohort of ethnically diverse children, ages 4 to 6 years old, living in Colorado. Mother-offspring dyads participating in the Healthy Start study have been followed from early pregnancy (before 24 weeks of gestation). Information on pregnancy and birth characteristics have been reported elsewhere 35,36. Written informed consent was obtained from the mother or legal guardian of the child. The protocol was approved by the Colorado Multiple Institution Review Board.
Study Visit Procedures
Participants (children) and their mothers or legal guardians were invited to the University of Colorado for two in-person visits. At the first in-person visit, trained research professionals conducted the Eating in the Absence of Hunger test paradigm 37. Additional demographic, child growth and health data were collected. Brain scans, described below, were conducted at the second in-person visit, within 6 months of the first in-person visit.
The Eating in the Absence of Hunger Test Paradigm
To dissociate hedonically-motivated eating from consumption of food due to homeostatic hunger, eating behavior was measured under sated conditions 38, using the Eating in the Absence of Hunger test paradigm. Portion sizes for the lunch meal items and for the palatable snacks were derived from Fisher and Birch 37. Children were offered a standard lunch meal and instructed to eat until they felt full. The standard lunch meal included chicken nuggets (Kroger brand; 4 nuggets; 67 grams), a butter role (King Soopers store brand Hawaiian rolls; 1 roll; 27 grams), carrots (King Soopers store brand produce; 30 grams), raisins (Kroger brand raisin minis; 2 boxes; 28 grams per box), graham crackers (Kroger brand; 4 squares; 28 grams), and milk (Kroger brand; 8 ounces; 180 grams). Each lunch item was weighed before and after consumption. Lunch meal energy intake (kilocalories) was calculated by taking the difference in weight (grams) before and after consumption and multiplying this by the kilocalories per gram for the respective food item derived from manufacturers’ information. Additionally, children were asked to report on their “fullness” after finishing the lunch meal to confirm satiation using a pictorial representation of a person with their stomach colored-in, with the categories of empty (no color in stomach image), just right (stomach image colored most of the way), or really full (stomach image colored completely).
Thirty minutes after the lunch meal was completed, children were given the opportunity (10 minutes) to consume additional energy from an assortment of highly palatable snacks, hereafter referred to as eating in the absence of hunger (EAH). Snacks included both sweet and savory foods items: potato chips (Kroger brand; 58 grams), pretzels (Kroger brand; 39 grams), Skittles (66 grams), chocolate (Hershey; 66 grams), Oreo cookies (6 cookies; 70 grams), donuts (Little Debbie powdered; 4 donuts; 53 grams), and a Honey bun (Little Debbie; 1 bun; 50 grams). All snacks were presented without packaging and in separate containers or on separate dishes. EAH intake was measured and calculated as described above. The kilocalories consumed from these snacks (EAH kcal), an indicator of hedonically-motivated disinhibited eating behavior, represents the extent to which children consumed excess energy, despite satiation, when exposed to highly palatable foods.
Imaging Protocol
All scanning activities were conducted at the University of Colorado Brain Imaging Center. Prior to scanning, child participants were exposed to a mock scanner, which closely resembles the research scanner in size and sound. Mock scanning sessions were at least 10 minutes in length, during which children were placed on the bed platform and moved into the “bore”. The children wore headphones so that they could listen to the various sounds that a magnetic resonance imaging (MRI) scanner produces while taking images of the brain.
All images were acquired on a Siemens 3T Skyra scanner (Siemens, Erlangen, Germany). Whole brain resting state functional MRI (rs-fMRI) was measured over a 6-minute period using the Blood Oxygen Level Dependent (BOLD) signal (144 scans, TR = 2.5 s, TE = 34 ms, matrix 66 × 66, flip angle 80, voxel size 3 mm3). A T1- weighted anatomical scan (3D-MPRAGE, 176 slices, TR = 2.2 s, TE = 2.45 ms, matrix 256 × 256, voxel size 1 mm3) was also collected. The child’s head was stabilized within the coil using foam padding. A movie was projected for the duration of the scan period in an effort to reduce movement during the scanning session. Participants were allowed to choose the movie from a limited selection of age-appropriate Pixar films (e.g. Ice Age, Finding Nemo, Toy Story, Frozen). There was no significant food-related content in the movies provided. Sound was provided through MRI-compatible headphones. Total scan time including the rs-fMRI BOLD, T1, and additional anatomical sequences was 38 minutes.
Data Preprocessing
All scans were initially reviewed and assessed for movement. Participants with movement greater than 3 mm translation or 0.3 degrees rotation were excluded from further processing and analysis. rs-fMRI preprocessing was conducted in Statistical Parametric Mapping (SPM) 12 (Wellcome Trust Centre for Neuroimaging, London, UK). Participant rs-fMRI data were slice-time corrected to the image acquired at 50% of scan completion and realigned to the first volume. Volumes were then co-registered to the participant’s skull-stripped anatomical T1 scan, and spatial normalization completed via unified segmentation [25] using a study-specific pediatric tissue probability map in Montreal Neurological Institute (MNI)-152 space generated via Dartel in SPM 12 39 from 95 typically developing anatomical T1 scans acquired from the larger Healthy Start study cohort (average age of 5.5 years old; range 4 – 6 years old). Finally, images were smoothed using an 8-mm full-width half-maximum Gaussian kernel.
Data Analysis
For the purpose of the current study, inclusion in the analytic sample required completion of the Eating in the Absence of Hunger test paradigm and completion of a rs-fMRI scan as of September 2017 (n = 27). Nine children were further excluded from the analytic sample for excess movement, as defined above. This resulted in an analytic sample size of 18 children for the present analysis. The nine children who were excluded due to movement did not differ significantly by age, weight (kg), or EAH kcal consumed as compared to the 18 children who were included in the present analysis. However, among those excluded due to movement, a smaller proportion were female compared to those who were included (44% vs. 61%).
Within Network Intrinsic Neuronal Activity
Independent Component Analysis (ICA) was used to identify the networks of interest, including, the basal ganglia network (i.e. reward network), executive control network, default mode network (anterior and posterior), and salience network. ICA is a data reduction technique in which voxels within a whole brain volume are grouped into spatially distinct networks based on the shared temporal variance between the voxels’ signal time series [29]. Specifically, in this analysis, functional brain networks were identified and isolated using the Group ICA of fMRI Toolbox (GIFT) in MATLAB [28]. Twenty-eight independent components (IC) were identified via the minimum description length criteria. Components were extracted, and the functional networks of interest identified by spatially matching resultant component maps to age-appropriate Generation R pediatric templates [26], and to the Functional Imaging in Neuropsychaitric Disorders Lab template for the basal ganglia network, specifically 28. ICs that spatially matched to templates of interest at a correlation value of ≥0.30 were then examined in second-level statistical analyses in SPM 12.
In second-level analyses, eigenvalues from the selected network components of interest were used as the outcome variable, with EAH kcal as the main predictor in general linear regression models. Voxel clusters whose activity was significantly associated with EAH kcal within each functional network of interest were identified. Monte Carlo simulations (10,000 iterations) were implemented via 3dClustSim (AFNI v.16.2.6 with spatial autocorrelation function applied) to correct for multiple comparisons. Clusters within each of the network ICs tested containing at least 52 contiguous voxels at a peak p-value < 0.005 were declared significant at an α < 0.05.
Between-Network Intrinsic Functional Connectivity
One to two major nodes within each functional network were identified based on reverse inference maps in Neurosynth’s meta-analytic database (http://neurosynth.org/; keywords = “default mode network”, “salience network”, “reward”, “response inhibition”, “cognitive control”). Major nodes were set as seed regions for functional connectivity analysis. All nodes investigated were bilateral except for the anterior and posterior cingulate cortices, and medial prefrontal cortex. Specifically, the posterior cingulate cortex and medial prefrontal cortex were defined as nodes within the default mode network, and anterior cingulate cortex and insula as the nodes within the salience network. The basal ganglia network included the caudate, putamen, pallidum, and nucleus accumbens as the major nodes. The superior, middle and inferior (pars triangularis and opercularis) frontal gyri and frontal poles were specified as major nodes of the executive control network. Each seed region was also considered as a target region for inter-network connectivity, resulting in a 23×23 connectivity matrix.
Using the defined seed regions, a seed-based region of interest (ROI)-to-ROI analysis was conducted via the CONN toolbox in MATLAB 40. The BOLD signal was denoised with linear detrending and despiking before modeling within-subject connectivity (i.e., first-level analysis). Regressors of no interest in the first-level analyses included cerebral spinal fluid and white matter signal, as well as realignment parameters. A multivariable general linear regression model was used in second-level analyses to test the association between the amount of EAH kcal consumed and functional connectivity between seed and target regions, while adjusting for a priori defined covariates, child age (months) and sex. Significance was defined at p < 0.05 with False Discovery Rate-correction.
RESULTS
The eighteen children included in the analytic sample were on average 5.8 years old (SD = 0.5 years), non-obese (body mass index z-score = −0.63, SD = 0.9, range: −2.5 – 1.0), 61% female, and consumed a mean of 156 EAH kcals (SD = 86.7 EAH kcals). The majority of children (72%) reported their fullness level as “just right” or “really full” after the lunch meal. Two children were identified as having consumed less than 50 EAH kcals, both of which had rated their fullness level after the lunch meal as “just right”. There was an additional outlier on the upper end of the EAH kcal distribution where one child had consumed over 300 EAH kcals. This child had also rated his/her fullness level after the lunch meal as “just right”. More detailed demographics are available in Table 1.
Table 1.
Descriptive characteristics of children included in analytic sample.
| Analytic Sample (n = 18) |
|
|---|---|
| Age (years), mean (SD), range | 5.8 (0.5) 5.2 – 6.7 |
| Sex, n (%): | |
| Male | 7 (38.9) |
| Female | 11 (61.1) |
| Race/Ethnicity, n (%): | |
| Non-Hispanic white | 11 (61.1) |
| Non-Hispanic black | 3 (16.7) |
| Hispanic | 4 (22.2) |
| Other | 0 |
| Weight (kg), mean (SD), range | 17.1 (2.0) 12.8 – 21.6 |
| BMI z-score, mean (SD), range | −0.63 (0.9) −2.5 – 1.0 |
| Household Income, n (%): | |
| < $50,000 per year | 8 (44.4) |
| ≥ %50,000 per year | 10 (55.6) |
| Lunch kcals, mean (SD), range | 165.2 (122.3) 10.0 – 441.9 |
| EAH kcals, mean (SD), range | 155.7 (86.7) 11.0 – 305.2 |
| Fullness rating, n (%): | |
| Empty | 2 (11) |
| Just right | 11 (61) |
| Really full | 5 (28) |
Within Network Intrinsic Neuronal Activity
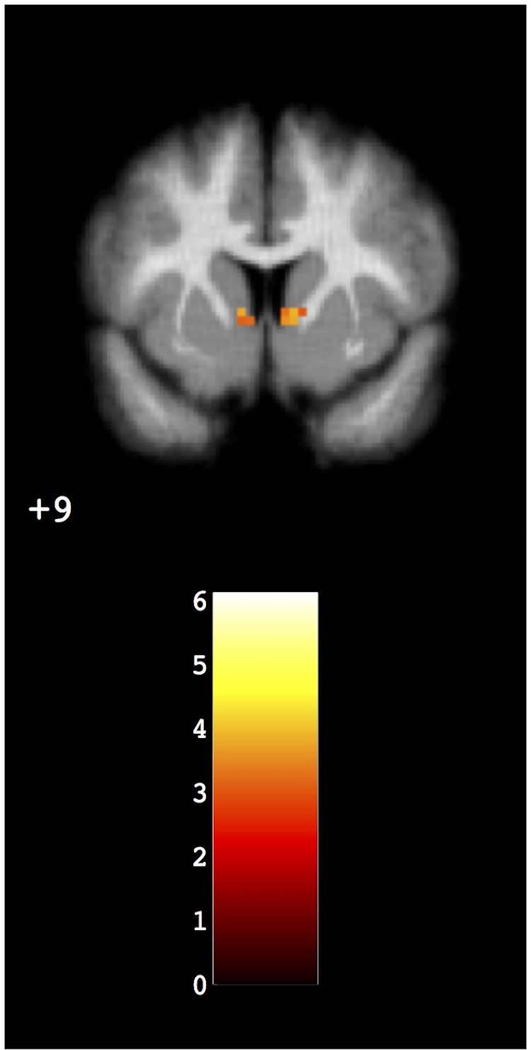
The amount of EAH kcals consumed was positively associated with neuronal activity in the bilateral nucleus accumbens (basal ganglia/reward network; x= −3, y= −7, z= 5; KE 79; t=6.12). See Figure 1. Clusters in the salience network, default mode network and executive control network did not survived threshold correction (p < 0.05 corrected).
Figure 1.
Within-network neuronal activity. Greater amount of EAH kcals consumed was associated with neuronal hyperactivity in the bilateral nucleus accumbens.
Between-Network Intrinsic Functional Connectivity
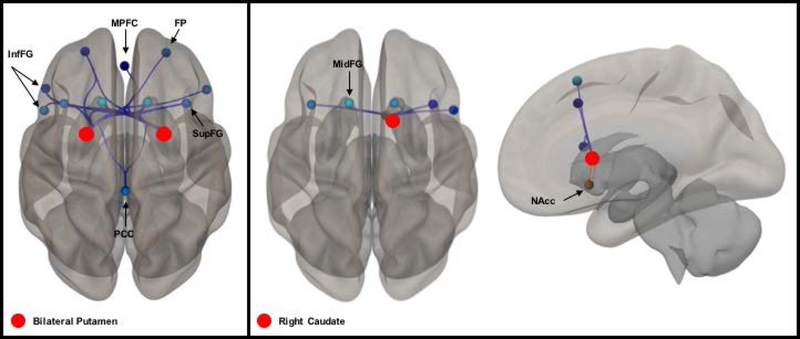
Connectivity patterns between the salience and basal ganglia networks were not significantly associated with EAH kcal, therefore, the hypothesis that connectivity between the salience and basal ganglia networks would be positively associated with eating in the absence of hunger was not supported. Connectivity between the right caudate of the basal ganglia network (F4,11 = 5.61, p < 0.05 corrected) and executive control nodes (bilateral superior and middle frontal gyri, and right inferior frontal gyri) was significantly and inversely associated with EAH kcal. Similarly, connectivity between the bilateral putamen of the basal ganglia network (F4,11 = 8.97 [left] and 8.19 [right], p < 0.01 corrected for both, respectively) and executive control nodes (bilateral frontal pole; bilateral superior, inferior, and middle frontal gyri) was inversely associated with EAH kcal. These data provide support for the hypothesis that basal ganglia and executive control networks would be negatively associated with eating in the absence of hunger. Bilateral putamen connectivity with the default mode network nodes (medial prefrontal cortex, posterior cingulate gyrus) was also inversely associated with EAH kcal. See Figure 2A. Connectivity within the basal ganglia network, specifically between the right caudate and the right nucleus accumbens, was positively associated with EAH kcal (t = 3.19, p < 0.05 corrected; Figure 2B).
Figure 2.
Between-network connectivity is inversely associated with EAH kcal. Red spheres denote seed-nodes. Blue lines represent an inverse association between connectivity and EAH kcal. Orange lines represent a positive association between connectivity and EAH kcal. Darkness or lightness of coloring in target-nodes reflects greater or lesser magnitude of the association with EAH kcal. (A) Connectivity between the bilateral putamen of the basal ganglia network and bilateral frontal pole (FP), superior (SupFG) and inferior (InfFG) frontal gyri of the executive control network, and posterior cingulate (PCC) and medial prefrontal cortex (MPFC) of the default mode network; (B) Connectivity between the right caudate and nucleus accumbens (NAcc) of the basal ganglia network, and SupFG and InfFG of the executive control network.
DISCUSSION
To the authors’ knowledge, this is the first study to investigate the relationship between observed hedonically-motivated disinhibited eating behavior and neuronal activity within the executive control, salience, reward and default mode networks and connectivity between these networks in typically developing young children without obesity. The results of the current study confirm observations made by prior studies in which hedonically-motivated disinhibited eating behaviors were documented in young children, 3–6 years-old 41–44. This study also provides new evidence of neuronal correlates of disinhibited eating behaviors in this young age group, indicating that alterations in executive control, salience, reward and default network functional circuitry begin early in the life course and may underlie the risk for later life obesity via overeating. Furthermore, some evidence suggests that eating in the absence of hunger, as a specific disinhibited eating behavior, is stable over time 43. Thus, children who demonstrate hedonically-motivated disinhibited eating early in childhood may continue to overeat in this manner. Multiple studies have also shown that eating in the absence of hunger behavior increases with age 45,46, further compounding the risk of diet-induced obesity into adolescence. The current results, in conjunction with these studies, suggest that underlying neurobiological processes may contribute to initiating disinhibited eating behavior as well as promote their pervasiveness throughout the life course. However, longitudinal studies are needed to affirm the developmental pathway from alterations in brain function to presentation of disinhibited eating behaviors.
This study’s results are consistent with one other study in older children where neuroimaging was implemented, and disinhibited eating behaviors measured. Specifically, Boutelle et al. found a significant and positive correlation between kilocalories consumed during the eating in the absence of hunger paradigm and bilateral neuronal activity in the striatum and insula among 8 to 13-year-old children with obesity compared to children without obesity 15. Interestingly, in contrast to the intrinsic “resting-state” methods employed in the current study, Boutelle et al. measured neuronal activity in response to administering a sucrose solution. The consistency between the current study’s finding of intrinsic (at-rest) hyperactivity in the nucleus accumbens in young children without obesity and Boutelle’s parallel finding in children with obesity in response to a sweet taste suggests that, 1) the relationship between reward neural circuits and hedonically-motivated disinhibited eating behavior can be observed in the absence of a food-related stimulus, and 2) this relationship is present prior to obesity development, providing evidence that the underlying neurobiology of disinhibited eating behavior is not necessarily a consequence of obesity. However, the current results need replication in other longitudinal pre-birth pediatric cohorts to affirm the developmental trajectory from altered neurobiology to disinhibited eating behaviors, and ultimately to obesity.
The current study’s results are also consistent with others who have found reduced connectivity between brain regions sub-serving reward (e.g. nucleus accumbens) and response inhibition in children who demonstrate greater food approach behaviors. Among children having a full spectrum of BMI values, Chodkowski et al. found an inverse association between connectivity between the nucleus accumbens and inferior parietal lobe (e.g. response inhibition) and parent-reported food approach behavior, specifically enjoyment of food 25. In other words, children reported to have a greater interest in food demonstrated weaker communication between the reward and response inhibition-related regions. Further, others have found that overeating is related to neuronal activity in inhibitory control regions, as shown by Adise et al. in 7 to 11-year old children where they showed a significant correlation between BOLD response to a reward task in the dorsolateral prefrontal cortex and amount of food consumed during a palatable buffet 47. These data suggest that neurobiological alterations in brain activity and connectivity may prime children for disinhibited eating behaviors via reward reactivity and inhibitory deficits that promote greater enjoyment and consumption of food.
In the current study, an inverse relationship was observed between eating in the absence of hunger and corticostriatal connectivity, specifically between bilateral putamen, right caudate (striatal) and the bilateral superior and inferior frontal gyri and frontal poles (cortical). While these corticostriatal connectivity results did not include the nucleus accumbens, the striatal regions identified in the current study do subserve cognitive/executive control processes 48, and suggest that weaker communication between executive control regions and the striatum may contribute to overeating of highly palatable foods. This putative role of executive function in eating behaviors is consistent with other studies suggesting cognitive factors are linked to disinhibited eating behaviors and obesity 49–51. However, the current study was cross-sectional in design and, therefore, the causality of the relationship between alterations in brain activity and connectivity in executive control regions and eating behaviors cannot be confirmed from these data.
Together with the other pediatric studies 15,47, the current results further implicate altered top-down control in hedonically-motivated disinhibited eating behavior in young children. Importantly, the current study is unique in that it employed an observed measure of disinhibited eating behavior, methodology that is not often implemented in neuroimaging studies of eating behaviors. The current study also investigated the association between brain function and eating behavior in non-obese, young children, therefore, establishing temporality of disinhibited eating behaviors and their underlying neurobiology before obesity development. Despite these strengths, this study’s results should be interpreted within the context of the limitations of the data. In particular, the gap in time between measurement of eating in the absence of hunger and the rs-fMRI (~6 months) was potentially large enough that developmental changes in both eating behavior and brain function may have occurred in this young age group. Fullness was also only assessed after the lunch meal ended. Given the 30 minutes between the end of the lunch meal and the beginning of the EAH test meal, children may have become fuller or hungrier. In future work, fullness should be measured both after lunch has ended and immediately before the EAH test meal is initiated to ensure the child’s state of satiety. Additionally, two children had rated their fullness level as “empty” after the lunch meal, implying that the EAH kcals that they consumed were not truly consumed in the absence of hunger. To address the potential influence of these outliers, sensitivity analyses were completed where these two children were removed from the sample and, as expected, the results did not change.
Finally, children were allowed to watch a movie during the scanning sessions which may have affected resting state brain activity or connectivity. While connectivity between the visual, frontal control, and dorsal attention networks has been shown to change in response to passive viewing of a movie as compared to no movie in young children 52, in our study, the overall effect of visual stimulation via movie watching would be consistent across all children, as all children watched a movie. However, it is possible that children who demonstrated greater eating in the absence of hunger could have also had greater reward sensitivity to the movie that they chose to watch. Therefore, the effect of this may have resulted in biasing the observed relationship.
In conclusion, the current study found evidence of an association between neuronal activity and connectivity in the reward and executive control systems of the brain and hedonically-motivated eating behavior in young children without obesity. These neuronal activity and connectivity patterns may represent pre-cursors or risk identifiers for future obesity development. Follow-up of the Healthy Start cohort will help to address the significance of the results found in current study in the development of childhood obesity. As the first of their kind, these results warrant further investigation into the potential insults or other developmental factors that may contribute to the neuronal activity and connectivity patterns that were identified in the current study.
ACKNOWLEDGEMENTS
Foremost, we would like to thank the parents and children who participated in the Healthy Start study. We would also like to thank the Pediatric Imaging Program at Children’s Hospital Colorado and the Department of Radiology, in collaboration with the Brain Imaging Center at University of Colorado, Anschutz for assistance in scanning Healthy Start participants. The Healthy Start study is funded by the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK076648, DD). The current work was funded, in part, by the National Institute of Health grants T32MH015442 (ALBS), and S10OD018435, R01DK103691, R01MH102224, R21DK102052 (JRT).
DD designed the parent Healthy Start cohort. ALBS, BS and JRT conceived of the analysis and ALBS carried out the analysis. SLJ conceived of and carried-out the Eating in the Absence of Hunger (EAH) paradigm and advised on the analytic approach to the EAH data. ALBS wrote the first draft of the manuscript and all authors were involved in reviewing and contributing significant scientific revisions to the final submitted manuscript.
ABBREVIATIONS
- EAH
Eating in the Absence of Hunger
- rs-fMRI
resting-state Functional Magnetic Resonance Imaging
- BOLD
Blood Oxygen Level Dependent
- SPM
Statistical Parametric Mapping
- MNI
Montreal Neurological Institute
- ICA
Independent Component Analysis
- IC
Independent Component
- GIFT
Group ICA of fMRI Toolbox
- ROI
Region of Interest
Footnotes
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Skinner AC, Ravanbakht SN, Skelton JA, Perrin EM, Armstrong SC. Prevalence of Obesity and Severe Obesity in US Children, 1999–2016. Pediatrics 2018. [DOI] [PMC free article] [PubMed]
- 2.Simmonds M, Llewellyn A, Owen CG, Woolacott N. Predicting adult obesity from childhood obesity: a systematic review and meta-analysis. Obesity reviews : an official journal of the International Association for the Study of Obesity 2016;17(2):95–107. [DOI] [PubMed] [Google Scholar]
- 3.Tanofsky-Kraff M, Shomaker LB, Olsen C, et al. A prospective study of pediatric loss of control eating and psychological outcomes. Journal of abnormal psychology 2011;120(1):108–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tanofsky-Kraff M, Cohen ML, Yanovski SZ, et al. A prospective study of psychological predictors of body fat gain among children at high risk for adult obesity. Pediatrics 2006;117(4):1203–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shomaker LB, Tanofsky-Kraff M, Elliott C, et al. Salience of loss of control for pediatric binge episodes: does size really matter? The International journal of eating disorders 2010;43(8):707–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Webber L, Hill C, Saxton J, Van Jaarsveld CH, Wardle J. Eating behaviour and weight in children. International journal of obesity (2005) 2009;33(1):21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lansigan RK, Emond JA, Gilbert-Diamond D. Understanding eating in the absence of hunger among young children: a systematic review of existing studies. Appetite 2015;85:36–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He J, Cai Z, Fan X. Prevalence of binge and loss of control eating among children and adolescents with overweight and obesity: An exploratory meta-analysis. The International journal of eating disorders 2017;50(2):91–103. [DOI] [PubMed] [Google Scholar]
- 9.Kral TV, Allison DB, Birch LL, Stallings VA, Moore RH, Faith MS. Caloric compensation and eating in the absence of hunger in 5- to 12-y-old weight-discordant siblings. The American journal of clinical nutrition 2012;96(3):574–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moens E, Braet C. Predictors of disinhibited eating in children with and without overweight. Behaviour research and therapy 2007;45(6):1357–1368. [DOI] [PubMed] [Google Scholar]
- 11.Hill C, Llewellyn CH, Saxton J, et al. Adiposity and ‘eating in the absence of hunger’ in children. International journal of obesity (2005) 2008;32(10):1499–1505. [DOI] [PubMed] [Google Scholar]
- 12.Butte NF, Cai G, Cole SA, et al. Metabolic and behavioral predictors of weight gain in Hispanic children: the Viva la Familia Study. The American journal of clinical nutrition 2007;85(6):1478–1485. [DOI] [PubMed] [Google Scholar]
- 13.Giskes K, van Lenthe F, Avendano-Pabon M, Brug J. A systematic review of environmental factors and obesogenic dietary intakes among adults: are we getting closer to understanding obesogenic environments? Obesity reviews : an official journal of the International Association for the Study of Obesity 2011;12(5):e95–e106. [DOI] [PubMed] [Google Scholar]
- 14.Schachter S Obesity and eating. Internal and external cues differentially affect the eating behavior of obese and normal subjects. Science 1968;161(3843):751–756. [DOI] [PubMed] [Google Scholar]
- 15.Boutelle KN, Wierenga CE, Bischoff-Grethe A, et al. Increased brain response to appetitive tastes in the insula and amygdala in obese compared with healthy weight children when sated. International journal of obesity (2005) 2015;39(4):620–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bruce AS, Holsen LM, Chambers RJ, et al. Obese children show hyperactivation to food pictures in brain networks linked to motivation, reward and cognitive control. International journal of obesity (2005) 2010;34(10):1494–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davids S, Lauffer H, Thoms K, et al. Increased dorsolateral prefrontal cortex activation in obese children during observation of food stimuli. International journal of obesity (2005) 2010;34(1):94–104. [DOI] [PubMed] [Google Scholar]
- 18.English LK, Fearnbach SN, Lasschuijt M, et al. Brain regions implicated in inhibitory control and appetite regulation are activated in response to food portion size and energy density in children. International Journal Of Obesity 2016;40:1515. [DOI] [PubMed] [Google Scholar]
- 19.Stice E, Yokum S, Burger KS, Epstein LH, Small DM. Youth at risk for obesity show greater activation of striatal and somatosensory regions to food. The Journal of neuroscience : the official journal of the Society for Neuroscience 2011;31(12):4360–4366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rolls ET. Taste, olfactory, and food texture processing in the brain, and the control of food intake. Physiology & behavior 2005;85(1):45–56. [DOI] [PubMed] [Google Scholar]
- 21.Small DM, Voss J, Mak YE, Simmons KB, Parrish T, Gitelman D. Experience-dependent neural integration of taste and smell in the human brain. J Neurophysiol 2004;92(3):1892–1903. [DOI] [PubMed] [Google Scholar]
- 22.Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain structure & function 2010;214(5–6):655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu X, Hairston J, Schrier M, Fan J. Common and distinct networks underlying reward valence and processing stages: a meta-analysis of functional neuroimaging studies. Neuroscience and biobehavioral reviews 2011;35(5):1219–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sescousse G, Caldu X, Segura B, Dreher JC. Processing of primary and secondary rewards: a quantitative meta-analysis and review of human functional neuroimaging studies. Neuroscience and biobehavioral reviews 2013;37(4):681–696. [DOI] [PubMed] [Google Scholar]
- 25.Chodkowski BA, Cowan RL, Niswender KD. Imbalance in Resting State Functional Connectivity is Associated with Eating Behaviors and Adiposity in Children. Heliyon 2016;2(1):e00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park BY, Seo J, Park H. Functional brain networks associated with eating behaviors in obesity. Sci Rep 2016;6:23891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Batterink L, Yokum S, Stice E. Body mass correlates inversely with inhibitory control in response to food among adolescent girls: an fMRI study. NeuroImage 2010;52(4):1696–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shirer WR, Ryali S, Rykhlevskaia E, Menon V, Greicius MD. Decoding Subject-Driven Cognitive States with Whole-Brain Connectivity Patterns. Cerebral Cortex 2012;22(1):158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wijngaarden MA, Veer IM, Rombouts SA, et al. Obesity is marked by distinct functional connectivity in brain networks involved in food reward and salience. Behavioural brain research 2015;287:127–134. [DOI] [PubMed] [Google Scholar]
- 30.Zhao J, Li M, Zhang Y, et al. Intrinsic brain subsystem associated with dietary restraint, disinhibition and hunger: an fMRI study. Brain imaging and behavior 2017;11(1):264–277. [DOI] [PubMed] [Google Scholar]
- 31.Kullmann S, Heni M, Veit R, et al. The obese brain: association of body mass index and insulin sensitivity with resting state network functional connectivity. Human brain mapping 2012;33(5):1052–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kullmann S, Pape AA, Heni M, et al. Functional network connectivity underlying food processing: disturbed salience and visual processing in overweight and obese adults. Cerebral cortex (New York, NY : 1991) 2013;23(5):1247–1256. [DOI] [PubMed] [Google Scholar]
- 33.Tuulari JJ, Karlsson HK, Hirvonen J, Salminen P, Nuutila P, Nummenmaa L. Neural circuits for cognitive appetite control in healthy and obese individuals: an fMRI study. PloS one 2015;10(2):e0116640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cornier MA, McFadden KL, Thomas EA, Bechtell JL, Bessesen DH, Tregellas JR. Propensity to obesity impacts the neuronal response to energy imbalance. Front Behav Neurosci 2015;9:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Starling AP, Brinton JT, Glueck DH, et al. Associations of maternal BMI and gestational weight gain with neonatal adiposity in the Healthy Start study. The American Journal of Clinical Nutrition 2015;101:302–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shapiro AL, Kaar JL, Crume TL, et al. Maternal diet quality in pregnancy and neonatal adiposity: the Healthy Start Study. Int J Obes (Lond) 2016;40(7):1056–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fisher JO, Birch LL. Restricting access to foods and children’s eating. Appetite 1999;32(3):405–419. [DOI] [PubMed] [Google Scholar]
- 38.Lowe MR, Butryn ML. Hedonic hunger: a new dimension of appetite? Physiology & behavior 2007;91(4):432–439. [DOI] [PubMed] [Google Scholar]
- 39.Ashburner J A fast diffeomorphic image registration algorithm. NeuroImage 2007;38(1):95–113. [DOI] [PubMed] [Google Scholar]
- 40.Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect 2012;2(3):125–141. [DOI] [PubMed] [Google Scholar]
- 41.Birch LL, Fisher JO. Mothers’ child-feeding practices influence daughters’ eating and weight. The American journal of clinical nutrition 2000;71(5):1054–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Faith MS, Berkowitz RI, Stallings VA, Kerns J, Storey M, Stunkard AJ. Eating in the absence of hunger: a genetic marker for childhood obesity in prepubertal boys? Obesity (Silver Spring, Md) 2006;14(1):131–138. [DOI] [PubMed] [Google Scholar]
- 43.Fogel A, McCrickerd K, Fries LR, et al. Eating in the absence of hunger: Stability over time and associations with eating behaviours and body composition in children. Physiology & behavior 2018. [DOI] [PMC free article] [PubMed]
- 44.Miller AL, Gearhardt AN, Retzloff L, Sturza J, Kaciroti N, Lumeng JC. Early Childhood Stress and Child Age Predict Longitudinal Increases in Obesogenic Eating Among Low-Income Children. Acad Pediatr 2018. [DOI] [PMC free article] [PubMed]
- 45.Francis LA, Ventura AK, Marini M, Birch LL. Parent overweight predicts daughters’ increase in BMI and disinhibited overeating from 5 to 13 years. Obesity (Silver Spring, Md) 2007;15(6):1544–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shunk JA, Birch LL. Girls at risk for overweight at age 5 are at risk for dietary restraint, disinhibited overeating, weight concerns, and greater weight gain from 5 to 9 years. Journal of the American Dietetic Association 2004;104(7):1120–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Adise S, Geier CF, Roberts NJ, White CN, Keller KL. Is brain response to food rewards related to overeating? A test of the reward surfeit model of overeating in children. Appetite 2018;128: 167–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Balleine BW, Delgado MR, Hikosaka O. The role of the dorsal striatum in reward and decision-making. The Journal of neuroscience : the official journal of the Society for Neuroscience 2007;27(31):8161–8165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dohle S, Diel K, Hofmann W. Executive functions and the self-regulation of eating behavior: A review. Appetite 2018;124:4–9. [DOI] [PubMed] [Google Scholar]
- 50.Francis LA, Susman EJ. Self-regulation and rapid weight gain in children from age 3 to 12 years. Archives of pediatrics & adolescent medicine 2009;163(4):297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Graziano PA, Calkins SD, Keane SP. Toddler self-regulation skills predict risk for pediatric obesity. International journal of obesity (2005) 2010;34(4):633–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Emerson RW, Short SJ, Lin W, Gilmore JH, Gao W. Network-level connectivity dynamics of movie watching in 6-year-old children. Frontiers of human neuroscience 2015;9:631. [DOI] [PMC free article] [PubMed] [Google Scholar]